For over four decades, polls in the USA have demonstrated that nearly 70% of smokers want to quit and 55% of smokers have attempted to quit in the past year.1 Over these decades, despite advances in medications and behavioural treatments, less than 10% of smokers successfully quit.2 Sadly, an estimated 31 million adult Americans continue to smoke and 11 million are e-cigarette users.3,4
In the USA, there are currently seven United States Food and Drug Administration approved medications for tobacco addiction.5 Five medications are nicotine replacement products: nicotine polacrilex gum and lozenges, transdermal nicotine patches, a nicotine nasal spray and a nicotine inhaler. Two medications are non-nicotine-based: bupropion and varenicline. The most clinically successful single medication has been varenicline, which was approved 17 years ago in 2006.5 With peak global sales of US$1.1 billion, varenicline, brand named Chantix® (Pfizer, New York, NY) in the USA and Champix® (Pfizer, New York, NY) outside the USA, was voluntarily withdrawn from market by the manufacturer in 2021.6 Whilst effective, only 24% of patients completed their full 12-week course of varenicline and many reported that side effects were the principal reason for non-compliance.7 Since no new tobacco treatment medications have been approved in almost two decades, there is a desperate need for new effective medications with a benign side-effect profile.
Cytisinicline, a naturally derived, plant-based medication is a potential candidate to fill that void. A similar product called cytisine (Tabex®; Sopharma Pharmaceuticals, Sofia, Bulgaria) has been sold in Europe for over 20 years and has been utilized by over 4 million smokers, evidencing a strong safety profile in Europe.8 To date, more than 10,000 patients have participated in cytisinicline clinical trials worldwide, including phase III trials.
Activation of the mesolimbic dopaminergic system (a central nervous system circuit), specifically the nucleus accumbens and the ventral tegmental area, is believed to be the neural mechanism associated with the reinforcement and reward experienced by smoking.9 Similar to varenicline, cytisinicline is a partial agonist, partial antagonist for the alpha-4 beta-2 nicotinic acetylcholine (NAch) receptors in the brain, which are the same receptors that are activated by nicotine. When nicotine binds to the NAch receptors, dopamine is released. By weakly activating these receptors, cytisinicline can help to reduce the nicotine cravings and withdrawal symptoms that people often experience when they try to quit smoking. Simultaneously, cytisinicline acts as a partial antagonist and prevents nicotine from binding to the NAch receptors and releasing dopamine. This reduces the overall reward and satisfaction that nicotine provides, thus helping smokers provides, smokers to conquer their nicotine dependence.10–15
This review aims to highlight the latest data from clinical trials investigating the effectiveness of cytisinicline for smoking cessation.
The on-going research of cytisinicline for addiction-1 trial
There have been several clinical trials investigating the effectiveness of cytisinicline for smoking cessation in adult smokers.16–19 Achieve Life Sciences, Inc. (Bothell, WA, USA), a pharmaceutical company whose mission is the global development and commercialization of cytisinicline for smoking cessation and nicotine dependence, has attempted to redevelop and improve this product in their On-going Research of Cytisinicline for Addiction (ORCA) clinical trial programme.20
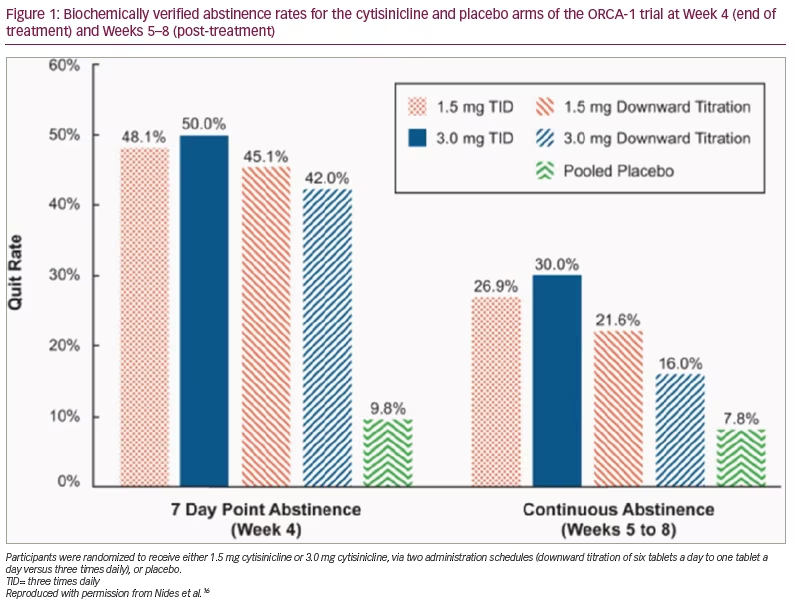
ORCA-1 is a double-blind, randomized, placebo-controlled clinical trial with five arms, which evaluated how a higher dosage and a simplified dosing schedule affected the efficacy and tolerability of cytisinicline (ClinicalTrials.gov identifier: NCT03709823).16 No previous studies have evaluated alternative cytisincline doses or administration schedules. For 25 days, participants received cytisinicline or placebo tablets plus behavioural support.16 Adult smokers (>10 cigarettes daily) who wished to quit smoking were randomized to receive either 1.5 mg or 3.0 mg cytisinicline, via two administration schedules (downward titration of six tablets a day to one tablet a day compared with three times daily [TID]). The secondary outcomes were the biochemically verified 7-day abstinence at Week 4 (end of treatment) and continuous abstinence from Weeks 5–8 post treatment; expired breath carbon monoxide (eCO) 7-day point-prevalence smoking abstinence rates were assessed at Week 4 and from Weeks 5–8 post treatment. Results were statistically significant, with higher abstinence rates (p<0.001) in all cytisinicline treatment arms compared with placebo. The quit rate was highest at 50% for the 3.0 mg cytisinicline TID arm compared with 10% for the placebo arms.16
Among the 254 randomized participants, those in the cytisinicline arms (regardless of dose or schedule) also had greater reductions in cigarettes smoked and had biochemically consistent reductions of 80% in eCO levels when compared with placebo arms.
All cytisinicline arms had statistically significantly higher abstinence rates at Week 4 compared with placebo and both TID cytisinicline arms demonstrated statistically significantly higher continuous abstinence rates from Weeks 5–8 compared with placebo (Figure 1).16 There were no significant safety concerns with either 1.5 mg or 3.0 mg cytisinicline administered using the TID schedule. The study concluded that participants in the 3.0 mg cytisinicline TID arm had the highest abstinence rate when using the increased dosage and easier administration schedule, compared with the 1.5 mg cytisinicline 25-day downward titration schedule that is marketed in Central and Eastern Europe.
The on-going research of cytisinicline for addiction-2 trial
Although a 1.5 mg cytisinicline 25-day titration schedule has been marketed in Europe for decades, 3.0 mg cytisinicline TID was selected by Achieve Life Sciences for phase III studies and potential market approval in the USA. This was due to the simpler dose scheduling, excellent tolerability and compliance, and higher continuous abstinence rate. The placebo-controlled ORCA-2 study (ClinicalTrials.gov identifier: NCT04576949) was the company’s first phase III study that evaluated the safety and efficacy of cytisinicline, dosed TID for a period of 6 or 12 weeks as a treatment for smoking cessation in 810 adult smokers.17,21 Patients were randomly assigned (1:1:1) to receive 3.0 mg cytisinicline TID for a period of either 6 or 12 weeks, or placebo. They were then monitored for smoking abstinence for 24 weeks post randomization. Behavioural support was provided for the duration of the trial.
The primary endpoint of the study was biochemically verified continuous abstinence measured during the last 4 weeks of the treatment period (Weeks 3–6 or Weeks 9–12). Results showed that both the 6- and 12-week cytisinicline treatments demonstrated significantly better quit rates than placebo, with odds ratios (OR) of 8.0 and 6.3, respectively (p<0.0001). Although participants were encouraged to attempt to quit smoking within the first week of treatment, the longer course of 12 weeks with continued quit attempts produced better quit rates. The abstinence rate during Weeks 9–12 was 32.6% for cytisinicline and 7.0% for placebo (Figure 2).21
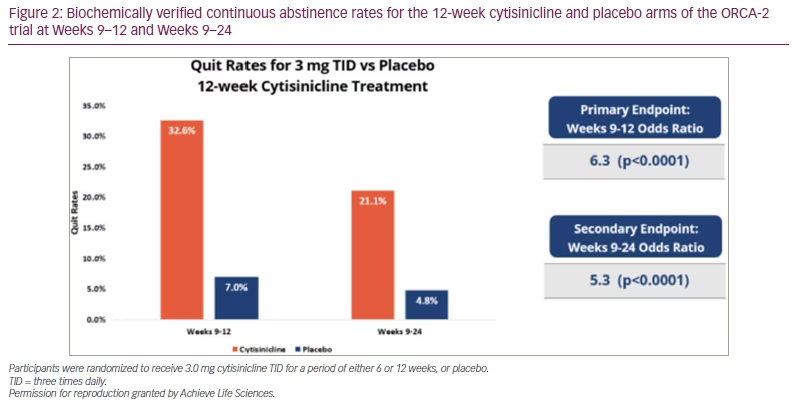
Secondary endpoints measured continuous abstinence after treatment through 24 weeks. Participants who received cytisinicline demonstrated significantly better quit rates than those who received placebo, at both the 6- and 12-week secondary endpoints. The Weeks 9–24 continuous abstinence rate for participants receiving 12 weeks of cytisinicline was 21.1%, compared with 4.8% for participants receiving 12 weeks of placebo (OR 5.3; p<0.0001). The Weeks 3–24 continuous abstinence rate for participants receiving 6 weeks of cytisinicline was 8.9%, compared with 2.6% for participants receiving 6 weeks of placebo (OR 3.7; p=0.0016).21
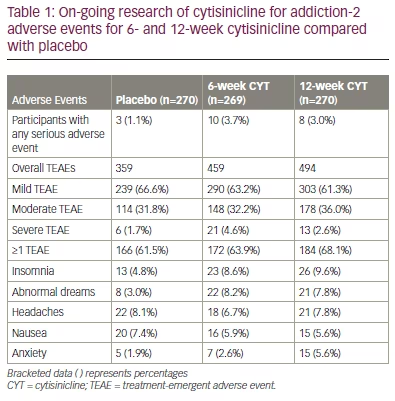
Cytisinicline, for both 6- and 12-week durations, was well tolerated and no treatment-related serious adverse events were reported (Table 1).21 Adverse events appeared mild and often similar to the rates observed with placebo. Cytisinicline has also been shown to be more than 2,000 times less potent than varenicline at binding to the human 5-hydroxytryptamine 3 receptor that causes nausea. This difference in 5-hydroxytryptamine 3 receptor binding is consistent with few nausea events for cytisinicline (even when compared with placebo) and when compared with the higher nausea rates for varenicline.19 No neuropsychiatric events other than anxiety were observed or reported. Anxiety was reported at 0.7% and 3.7% greater than placebo for 6-week and 12-week cytisinicline, respectively.
The on-going research of cytisinicline for addiction-3 trial
On 9 January 2023, Achieve Life Sciences announced that the treatment of the final participant of the phase III ORCA-3 trial (ClinicalTrials.gov identifier: NCT05206370) had concluded.18,22 Following the ORCA-2 trial, the ORCA-3 trial is evaluating the smoking cessation efficacy, safety and tolerability of either 6 or 12 weeks of 3.0 mg cytisinicline dosed TID, compared with placebo.18,22
The ORCA-3 trial has 792 participants across 20 clinical trial locations in the USA. In this anticipated final phase III registrational trial, after randomization participants will be monitored for 24 weeks and behavioural support will be provided for the trial period. Biochemically verified continuous abstinence during the last 4 weeks of treatment will be measured as the primary endpoint. An independent comparison of each treatment arm with the placebo arm will be conducted and the trial will be determined to be successful if either or both of the cytisinicline treatment arms show a statistical benefit compared with placebo. The results of ORCA-3 are anticipated to be released in the second quarter of 2023.22
Other supporting studies
Also supporting the ORCA phase III programme, a recent study has examined whether cytisinicline was at least as effective as varenicline as a smoking cessation medication in New Zealand’s indigenous Maori population, among which there is a high smoking prevalence (ClinicalTrials.gov identifier: NCT02957786).19 In the trial, 679 people were randomly assigned (1:1) to receive either 12 weeks of cytisinicline or 12 weeks of varenicline, with low- intensity behavioural support. The primary outcome was eCO confirmed continuous abstinence at 6 months. Secondary outcomes included eCO confirmed continuous abstinence at 1, 3, 6 and 12 months post-quit date, cigarettes per day in those who continued to smoke, adverse events, treatment compliance and acceptability. At 6 months post-quit date, the continuous abstinence rates were 12.1% for cytisinicline compared with 7.9% for varenicline (risk difference 4.3%, 95.0% confidence interval [CI] 0.22–8.79; relative risk 1.6, 95.0% CI 0.97–2.46). Self-reported adverse events occurred significantly less frequently over 6 months in the cytisinicline group (313 events in 111 participants for the cytisinicline group compared with 509 events in 138 participants for the varenicline group). Headache, nausea and difficulty sleeping were common adverse events, all of which were higher with varenicline.19
Whilst tobacco treatment specialists and other clinicians can and do use medications off-label, there are currently no approved treatments specifically indicated for e-cigarette or vaping cessation.23 Initiated in June 2022, Achieve Life Sciences is also investigating cytisinicline in the ORCA-V1 multicentre, double-blind, placebo-controlled study of daily adult nicotine e-cigarette vapers who intend to quit (ClinicalTrials.gov identifier: NCT05431387).23 Subject enrolment was completed in the fourth quarter of 2022.
Conclusion
Overall, cytisinicline is a promising tobacco treatment for adult smokers. It appears to be effective at reducing cravings and withdrawal symptoms, and may improve the chances of long-term abstinence from smoking. This clinician and author awaits Food and Drug Administration approval of the newly developed cytisinicline treatment regimen with optimistic anticipation. Hopefully, regulators in other countries will appreciate its efficacy and safety, approving cytisinicline for the world’s tobacco dependent.