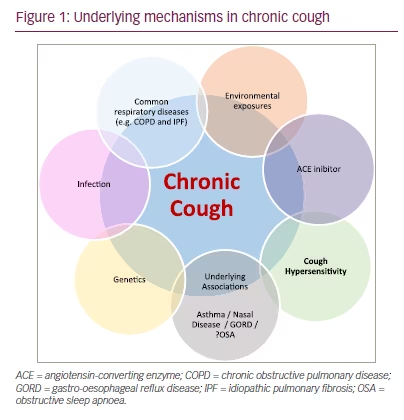
Chronic cough, defined as a cough lasting over 8 weeks, has an estimated global prevalence of 10% and has significant detrimental physical, social and psychological consequences on individuals.1,2 Chronic cough is a complex heterogeneous disease, in which many causative factors need to be explored (Figure 1).3 The three most reported associations are rhinosinusitis, gastro-oesophageal reflux disease and asthma.4 However, up to 59% of patients seen in specialist cough clinics have persistent symptoms despite treatment of these three triggers.5 Typically, these patients share similar characteristics, including an irritation or tickle in their throat and intense coughing to low-level stimuli (hypertussia), such as exposure to cold temperature, dry atmospheres and talking.6,7 This symptom set, alongside hypersensitivity and hyperresponsiveness to inhaled tussive agents compared with healthy volunteers, strongly suggest a neuropathic basis for the disorder.8 In this review we discuss the physiology of the chronic reflex and recent research in refractory/unexplained chronic cough (RUCC).
Overview of the cough neuroanatomy
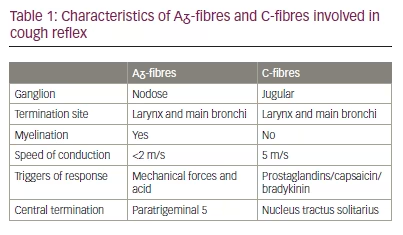
In humans, cough is under a combination of reflex and volitional control.9 Two categories of nerve fibres are primarily responsible for mediating cough: vagus Aᵹ-fibres and C-fibres (Table 1).10 Aᵹ-fibres are myelinated, thus have a fast speed of conduction (<2 m/s) and respond primarily to mechanical forces and acid. C-fibres, on the other hand, are unmyelinated, transmitting impulses at slower conduction speeds of 5 m/s but responding to a wider array of substances, including capsaicin, bradykinin and prostaglandin.11 Their cell bodies are in separate ganglia, nodose (Aᵹ-fibres) and jugular (C-fibres), and terminate in two slightly separate areas within the medulla oblongata of the brainstem.12 P2X purinoceptor 3 (P2X3) receptors are adenosine triphosphate (ATP)-activated ion channels located widely in the peripheral nervous system, including on both Aᵹ and C-fibres innervating the airways.13 Alongside other afferent nerve receptors, including transient receptor potential cation channel subfamily V member 1 (TRPV1) and TRPA1, they are thought to play a prominent role in cough neurophysiology.14 Supporting this concept, P2X3 antagonism has been shown to dramatically reduce cough frequency in patients with RUCC.15 In addition, cough control is influenced by the central nervous system, which can have both facilitatory and inhibitory effects on the cough reflex.9
Adenosine triphosphate and hypotonic saline challenge
Landmark trials demonstrating the efficacy of blocking the purinergic receptor, P2X3 (or heterotrimeric P2X2/3 receptor), in RUCC have sparked heightened interest in the mechanistic role of extracellular ATP in chronic cough.15,16 The mechanism in which ATP is released, or whether vagus afferent nerves are simply sensitized to ATP, is not known. Bonvini et al. have previously demonstrated that ATP release in guinea pigs occurs through an indirect mechanism involving TRPV4.13 In this model, hypotonicity (-80 Osm/L) activates TRPV4 receptors, leading to ATP release via the ion channel pannexin 1, and subsequent depolarization of Aᵹ-fibres (Figure 2).13
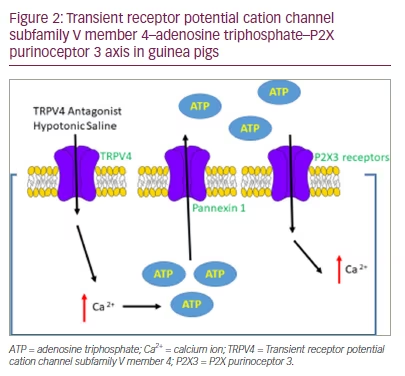
Whether a similar mechanism occurs in humans is unknown, but in our recent European Respiratory Society (ERS) abstract we demonstrate that hypotonic saline effectively discriminates RUCC from healthy volunteers.17 In this study, a typical cohort of participants with RUCC (n=38) were recruited and compared with healthy volunteers (n=38). Descending concentrations of hypotonic saline (300, 250, 200, 150, 100 mOsm/kg) were given in four single breath activations via a DeVilbiss 3000 ultrasonic nebulizer (DeVilbiss Healthcare Ltd., West Midlands, UK). The maximum number of coughs evoked at any given dose (Emax) was recorded for each participant.
Median (interquartile range) Emax was significantly higher in participants with RUCC (11.50 [4.75–24.25]) than in healthy volunteers (0.00 [0.00–2.00]; p<0.01). The observed cough frequency was higher across all concentrations in the RUCC group, but peaked at 150 mOsm/kg. Interestingly, in the RUCC group, the median (interquartile range) Emax was significantly higher in females (22.00 [6.00–30.00]) compared with males (6.00 [3.00–13.00]; p=0.02).
Hypotonic saline is a novel cough challenge that effectively discriminates RUCC from healthy volunteers. Compared with other cough challenge agents, such as capsaicin and citric acid, there is less overlap in evoked cough between health and disease.18 The observed differences are consistent with experiments in animal models, and suggest that the TRPV4–ATP–P2X3 axis may play a role in the pathophysiology of RUCC. Unfortunately, a small phase IIa study assessing the efficacy of a TRPV4 antagonist in RUCC showed no significant effect on cough frequency, but adequate dosing and effective target engagement were not demonstrated.19
Evidence for central mechanisms in refractory/unexplained chronic cough
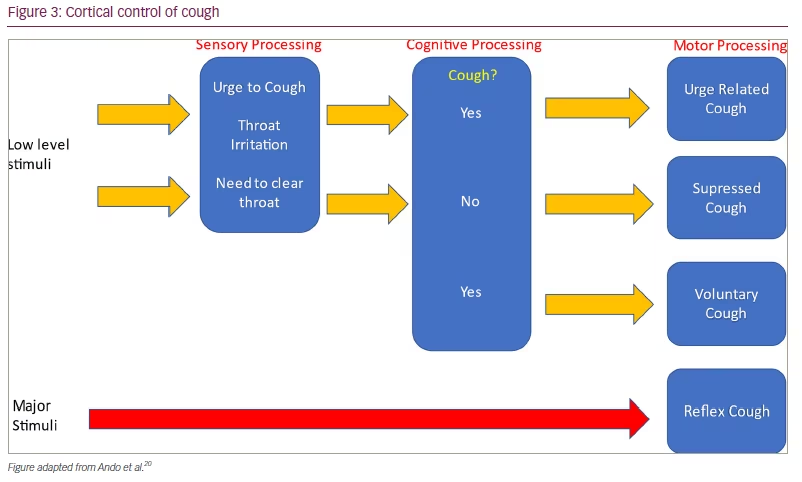
Our group have recently shown that compared with healthy volunteers, patients with RUCC have increased cough responses to a range of tussive challenge agents that stimulate receptors on both Aᵹ-fibres and C-fibres, including citric acid and capsaicin.18 This indiscriminate hypersensitivity suggests that, alongside factors stimulating peripheral nerves, central sensitization is implicated in the pathophysiology of RUCC. Central control of cough is also evident by varying behavioural responses to tussive stimuli (Figure 3),20 and further supported by the efficacy of centrally acting medications, such as opioids, in RUCC.21 Likewise, speech and language therapy, which is effective in RUCC, targets top-down control of cough, particularly the patient response to an urge to cough.22 Further evidence of central control of cough is seen with functional magnetic resonance imaging (fMRI). During simultaneous fMRI and capsaicin challenge, urge to cough was matched with higher blood oxygen level-dependent (BOLD) signal in midbrain processing regions, such as the periaqueductal grey and dorsal raphe nucleus, in RUCC compared with healthy volunteers.23 This heightened neural activity suggests that interoceptive pathways may be upregulated in RUCC, leading to increased responses to nociceptive stimuli. Interestingly, in the same study, patients with RUCC also had reduced BOLD responses in frontal network areas associated with suppression of motor reflexes. This suggests that both upregulated central nervous system processing and reduced cortical cough control may be implicated in the physiology of RUCC.
Pharmacological management of refractory/unexplained chronic cough
Despite a high burden of symptoms, treatment options are limited in RUCC. Low-dose morphine has been shown to lower cough frequency and improve patient-reported outcomes in RUCC,21,24 but there are concerns regarding tolerability of this medication, particularly considering the recent epidemic of opiate use in the United States.25 European guidelines do, however, recommend considering a trial of low-dose morphine (5–10 mg twice daily) in patients with RUCC under the care of a specialist cough clinic.26 Alternative options include gabapentin, pregabalin or amitriptyline, but the evidence for the efficacy of these medications is weaker.27–29
In our recent ERS abstract, we highlight the real-world effectiveness and tolerability of low-dose opiates in RUCC (n=100).30 In this retrospective review, the side effects and efficacy, as measured by a verbal (0–10) cough score, were recorded. The most used formulation and dose was 5 mg slow-release morphine sulphate tablets (MST) twice daily. We found that 75% of patients experienced some benefit in cough symptoms. Side effects did occur (38%), but could be managed with adjuncts or changes in formulation in the majority of patients (23 out of 38). Novel antitussives are needed to optimize care of patients with RUCC, but this study highlights acceptable tolerability of MST in this cohort, who were managed and closely observed under a specialist cough clinic.
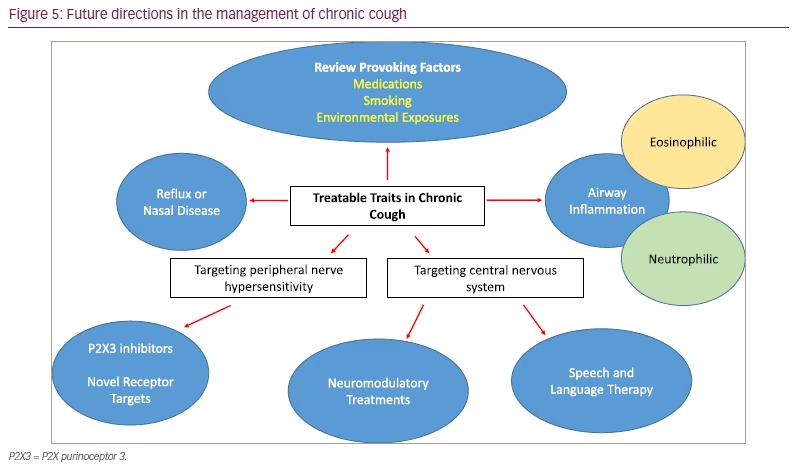
Targeting specific traits in chronic cough
International guidelines have highlighted the importance of identifying treatable traits in chronic cough,26 and previously set out investigation and treatment algorithms for common associations such as asthma, acid reflux and nasal disease (summarized in Table 2).26,31,32 There is less consensus on evaluation of treatment responses. There is a relationship between patient-reported outcomes (Leicester Cough Questionnaire and cough severity visual analogue scale) and 24-hour cough frequency, but the correlation is only weak to moderate in strength (p=0.30–0.58).33 These measures also do not help characterize specific traits in RUCC. Features of neuronal excitability, such as a background urge to cough and numerous cough triggers are common in RUCC, but not reported in all patients.7 The response of these cough characteristics to treatment has not been well described. Recently, the triggers and sensations provoking cough in different respiratory conditions were compared using a novel 49-item questionnaire.34 Interestingly, compared with other conditions, such as asthma, interstitial lung disease and bronchiectasis, patients with RUCC reported a greater number and severity of triggers and sensations associated with their cough. How these characteristics change over time and with treatment is an on-going area of intrigue.
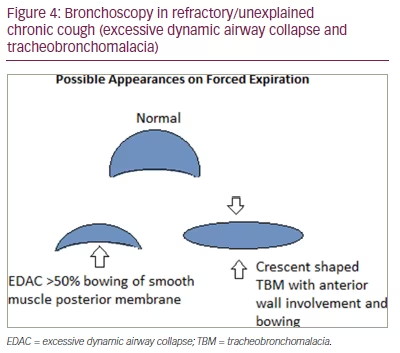
Another area of interest is patients with RUCC who display atypical characteristics, such as excessive sputum production (>1 tablespoon sputum daily) or an urge to cough associated with sensations located in the chest. These patients have historically been poorly characterized in the literature, despite making up to 21% of patients seen in specialist cough clinic.35 In this cohort, alongside cough hypersensitivity, additional factors should be considered and investigated such as: airway collapsibility (excessive dynamic airway collapse and tracheobronchomalacia; Figure 4), neutrophilic inflammation and eosinophilic bronchitis.36 These patients share similarities with non-cystic fibrosis bronchiectasis, where chest physiotherapy and daily nebulized hypertonic saline have been shown to improve patient-reported outcomes and lung function.37,38 Azithromycin, which can also be trialled in this cohort, has not been found to be broadly helpful in RUCC,39 but may offer benefit in subgroups with sputum production.40
Future treatments
Gefapixant is an investigational, orally administered drug that is being developed for the treatment of RUCC. The antitussive effects of gefapixant are thought to occur primarily through P2X3 antagonism, with taste side effects related to co-existing blockade of P2X2/3 receptors on the nerves innervating the tongue.41,42 In the COUGH-1 and COUGH-2 phase III studies, there was a high incidence of taste side effects, with 20% unable to continue gefapixant 45 mg twice daily due to adverse effects.43 It is therefore of interest that a recent phase IIa randomized placebo-controlled trial of the highly selective P2X3 antagonist, sivopixant, met the endpoint of demonstrating a significant reduction in placebo-adjusted average daytime cough frequency (-31%; p=0.0546), with patients reporting a lower incidence of taste side effects (12.9%).44 Having therapeutic options for targeting peripheral P2X3 receptors (selective and non-selective antagonists) and thereby reducing cough frequency will improve patient care, but centrally acting medications are also likely to remain important. Our review suggests that low-dose MST is well tolerated in RUCC, but there remains a low risk of addiction and side effects. Nalbuphine is an opiate that may negate this risk, as it is a dual-acting κ-opioid receptor agonist and µ-opioid receptor antagonist. It is therefore of interest that this medication is highly effective in cough associated with idiopathic pulmonary fibrosis, with a recent study demonstrating a significant reduction in placebo-adjusted hourly cough frequency using this medication (day 22 versus baseline, -51.6%; p<0.0001).45
Several mechanisms are likely to play a part in the presence of RUCC both within and between individuals. As described above, the response to individual antitussive agents is not uniform. The development of tools or biomarkers to predict an individual’s response to antitussive therapy would be an important development to reduce the burden of side effects and risks associated with pharmacological agents.
Conclusion
The mechanisms underpinning chronic cough are complex and multi-factorial, but our knowledge of neuronal hypersensitivity is increasing. The efficacy of P2X3 antagonists and cough reflex hypersensitivity and hyperresponsiveness to hypotonic saline suggest a role for extracellular ATP in this disorder. Future treatment may be multi-faceted, with treatment targeting control of underlying triggers and modulation of central and peripheral mechanisms leading to cough (Figure 5).