Asthma, classically defined as a chronic inflammatory disease of the lower respiratory tract, is a heterogeneous array of clinical disorders that differ in intensity, occurrence, triggers, comorbidities, genetics, clinical course and prognosis.1 It manifests as wheezing, cough, dyspnoea, chest tightness and fatigue.2 These manifestations can be episodic or permanent, and are usually associated with a variable, obstructive, bronchial airflow syndrome that is often reversible, either spontaneously or following treatment, though this reversibility may be incomplete in some patients with asthma.3
To date, there is no definite aetiology for asthma; however, many underlying risk factors have been established, such as tobacco smoke exposure, viral infections of the upper airway early in life, obesity, rhinitis, gastro-oesophageal reflux disease (GORD), income level and other socioeconomic factors, as well as genetics.4,5 In addition to bronchoconstriction and increased luminal secretions, combinations of the above factors result in mucosal inflammation of the lower airway, which is the pillar of the pathophysiology of asthma.3,6
The worldwide incidence of asthma in 2017 was 0.56% (43.12 million cases), and its prevalence in the same year was 3.57% (272.68 million cases); its mortality rate was 0.006% (0.49 million deaths).7 In Lebanon, the prevalence of current asthma in 2021 is 5%, and that of physician-diagnosed asthma is 6.7%, the latter being larger to the overall prevalence reported by the World Health Survey, which was 4.3%.8,9
Globally, asthma is a prominent cause of morbidity, high numbers of hospital admissions and high economic burden, especially for patients with severe disease.10 Asthma exacerbations are episodes of acute or subacute onset, characterized by heightened symptoms and reduced lung function that necessitate treatment intensification.11 In a multiple-country, retrospective cohort study, 8.4–12.5% of patients with asthma had at least one attack, and 1.7–2.5% experienced at least two attacks during the 12-month follow-up period, thus proving that exacerbations are frequent and can increase the risk of future exacerbations in the following 12 months.12 Exacerbations remain a significant source of illness-related morbidity, a major cause of healthcare consumption and hospitalizations, and a main factor in accelerating deterioration in lung function.13,14 Luskin et al. showed that exacerbations significantly decreased asthma-related quality of life and resulted in poorer Mini-Asthma Quality of Life Questionnaire (Mini-AQLQ) scores.15
The presence of multiple factors can affect the clinical presentation of exacerbations and their prognosis. McDonald et al. identified many pulmonary and non-pulmonary elements that increase exacerbation risk, such as eosinophilic pulmonary inflammation, vocal-cord dysfunction, depression and inhaler polymedication.16 Other inflammatory and comorbid factors include the presence of airway closure (predicted forced vital capacity [FVC] <80%), sputum eosinophils, hypertension, obstructive sleep apnoea and GORD.17
To the best of our knowledge, no such exhaustive studies have been conducted in the Lebanese population. Only one study identified the effect of obesity alone on asthma control, and concluded that high body mass index (BMI) was associated with poorer asthma control.18
Therefore, the aim of our study was to determine the predisposing factors for asthma exacerbation among Lebanese patients with asthma admitted to the emergency department and requiring subsequent hospitalization. We hypothesized that patients with asthma experiencing severe exacerbations differ from patients never hospitalized with asthma and have a particular phenotype, an epidemiological profile, a certain level of disease control, as well as clinical and/or biological predisposing factors that can be modified by therapy and/or lifestyle changes.
Methods
Data collection
Our study was a retrospective comparative study. We collected data on patients who were admitted to the pulmonology department at Hôtel-Dieu de France hospital, located in Beirut, between 2018 and 2020. These patients were designated as ‘inpatient’ in the statistical tables. Inclusion criteria were the following: patient >16 years old, diagnosed with asthma and under treatment, requiring hospitalization for a severe asthma exacerbation, with oxygen saturation (SpO2) <95% at the moment of admission or a decrease of 4% in SpO2 compared with baseline, with a life-threatening exacerbation, as defined by the Japanese Society of Allergology (inability to move due to dyspnoea, speaking difficulty).19 Exclusion criteria were the following: cardiac insufficiency, infectious pulmonary disease, sepsis, pneumothorax and advanced respiratory insufficiency. Forty patients were included in the hospitalized patients category and 10 were excluded due to the presence of one or more exacerbation factors.
In the control population, we collected data of 135 patients with controlled asthma, without prior exacerbation or hospital admission for asthma, from a pulmonology clinic in Beirut, Lebanon. These patients had several visits to the clinic between 2016 and 2020, and their asthma was stable. These patients were designated as ‘outpatient’ in the statistical tables.
Variables
Epidemiological factors were sex, BMI, level of education and habitat (rural or urban). Asthma-related factors were number of medicines used for asthma treatment, number of years since asthma diagnosis and blood levels of eosinophils. Patient comorbidities were divided into asthma comorbidities and cardiovascular comorbidities. Asthma comorbidities included were chronic sinusitis, allergic rhinitis, GORD, obstructive sleep apnoea and depression/anxiety. Cardiovascular risk factors and comorbidities included hypertension, diabetes and dyslipidaemia.
Asthma control prior to the exacerbation was assessed using the Global Initiative for Asthma (GINA) assessment of asthma control in adults, adolescents and children aged 6–11 years, which consists of four questions: presence of daytime asthma symptoms more than twice per week, nocturnal symptoms, need for reliever more than twice per week and activity limitation due to asthma (all of these in the 4 weeks preceding the exacerbation for hospitalized patients or at the time of presentation at the clinic for outpatients). A score of 1 is attributed to a positive response to any of the questions; a score of 0 indicates well-controlled asthma, a score of 1 or 2 indicates partly controlled asthma and a score of 3 or 4 indicates uncontrolled asthma.11,20
Statistical studies
The distribution of continuous variables was assessed using normality tests (Shapiro–Wilk) with visual inspection of the Quantile-Quantile plots. Ordinal variables and continuous variables in which distribution is skewed were expressed as median with interquartile range. Continuous data with a symmetric distribution were expressed as mean ± standard deviation. Categorical data were presented as frequencies, percentages and 95% confidence intervals (CIs).
Categorical data were compared between the two groups using chi-squared test, Fisher’s exact test, or Fisher–Halton–Freeman test, as appropriate. For Boolean variables, odds ratios and 95% CIs were calculated. Continuous variables with normal distribution were compared between the two groups using the independent samples T-test. Ordinal variables and continuous variables with skewed distribution were compared between the two groups using the non-parametric Mann–Whitney U test.
The statistical analysis was performed using the SPSS software (IBM Corp. Released 2019, SPSS Statistics for Windows Version 25.0, Armonk, NY, USA). The anonymized study dataset and the statistical code are available from the main author upon reasonable request.
Variables with a univariate p-value <0.05 were entered into a multivariate logistic regression model having the hospitalization as a binary dependent variable. A complete case approach was elected, and no imputations were tempted to replace missing values. The model’s calibration was evaluated (Omnibus Test of Model Coefficients, Log likelihood, Cox & Snell R², Hosmer and Lemeshow Test, C-statistic) and analogues of Cook’s distances and studentized residuals were checked. All the independent variables with a univariate p-value <0.05 were entered in the model, except for educational level, which was missing in more than 44% of the patients. The complete case approach yielded 85 observations (49%) available for analysis.
Ethical considerations
This study was approved by the ethics committee of Hôtel-Dieu de France hospital, Saint Joseph University. After deliberation, the committee voted that the study confirmed to ethical rules, and gave approval to perform the GINA questionnaire in patients. The committee acts in concordance with Good Clinical Practices described in the Helsinki Declaration and International Ethical Guidelines for Biomedical Research involving Human Subjects of the Council for International Organizations of Medical Sciences in collaboration of the World Health Organization. Participants provided non-written informed consent that was formally documented and witnessed prior to answering the questionnaire. The medical records were kept private and confidential.
RESULTS
Epidemiological factors
In our study, female sex, lower educational level, rural habitat, older age and higher BMI were independent risk factors for severe asthma exacerbation (Table 1). Both sexes were present in similar levels (45.9% males versus 54.1% females) in the outpatient group, whereas 80% (p=0.030) of inpatients were female (Table 1).
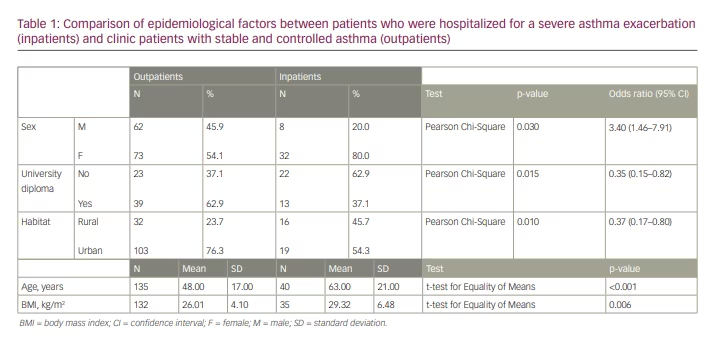
The mean age of inpatients (63 ± 21 years) was significantly greater than the mean age of outpatients (48 ± 17 years, p<0.001; Table 1). In addition, the BMI of inpatients (29.32 ± 6.48 kg/m2) was significantly greater than the BMI of outpatients (26.01 ± 4.10 kg/m2, p=0.006; Table 1).
Education level was also an important factor in asthma control. A university diploma was noted among 37.1% of inpatients compared with 62.9% (p=0.015) of outpatients (Table 1). The percentage of inpatients who lived in rural areas (45.7%) was significantly greater than for outpatients (23.7%, p=0.010; Table 1).
Asthma-related factors and comorbidities
Outpatients took more asthma medications than inpatients. The mean number of asthma medications for outpatients was 4 ± 1, whereas it was 3 ± 1 (p<0.001) for inpatients (prior to the exacerbation) (Table 2). The following asthma medications were considered: inhaled corticosteroids, long-acting β agonists, short-acting β agonists, long-acting muscarinic antagonists, leukotriene receptor antagonists, anti-immunoglobulin E antibodies and oral corticosteroids.
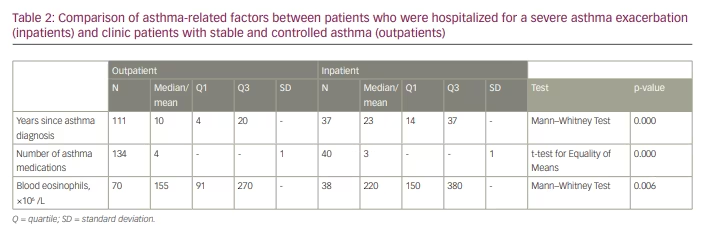
Our analysis showed that inpatients had asthma for more years than outpatients. The median number of years since asthma diagnosis was significantly greater among inpatients (23 years versus 10 years, respectively, p<0.001; Table 2).
Concerning blood tests, the median eosinophils level of inpatients (220 × 106 /L) was significantly greater than that of outpatients (155 × 106 /L, p=0.006; Table 2).
Obstructive sleep apnoea predisposed patients to asthma exacerbation with an odds ratio of 4.87. Its frequency among inpatients (28.2% [95% CI 16.0–43.5%]) was significantly greater than its frequency among outpatients (7.5% [95% CI 3.9–12.8%], p=0.001; Table 3). In addition, chronic sinusitis increased exacerbation risk with an odds ratio of 2.74 (25.6% [95% CI 14.0–40.7%] of inpatients versus 11.2% [95% CI 6.7–17.3%, p=0.024] of outpatients; Table 3). Results concerning allergic rhinitis and GORD, which are considered as asthma comorbidities, were not statistically significant.
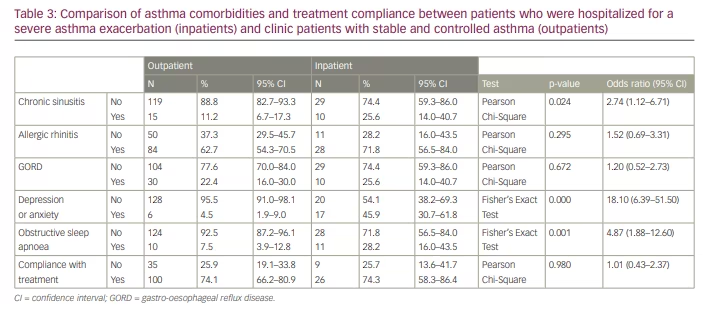
Psychological factors play an important role in severe exacerbations. Of inpatients, 45.9% (95% CI 30.7–61.8%) had anxiety or depression, compared with only 4.5% (95% CI 1.9–9.0%, p<0.001) of outpatients
(Table 3).
Compliance was evaluated based on the adherence to treatment as prescribed by the physician. Compliance among both categories was similar; 74% of patients in both categories were taking their treatment regularly or as prescribed by their physician, which was not a statistically significant difference (Table 3).
Hypertension, diabetes and dyslipidaemia were associated with asthma exacerbation requiring hospitalization. The use of tobacco did not significantly increase the risk of asthma exacerbation. Over half (57.5% [95% CI 42.1–71.9%]) of inpatients had hypertension compared with only 15.7% (95% CI 10.3–22.5%, p<0.001) of outpatients (Table 4). Hypertension predisposed patients to asthma exacerbation with an odds ratio of 7.28.
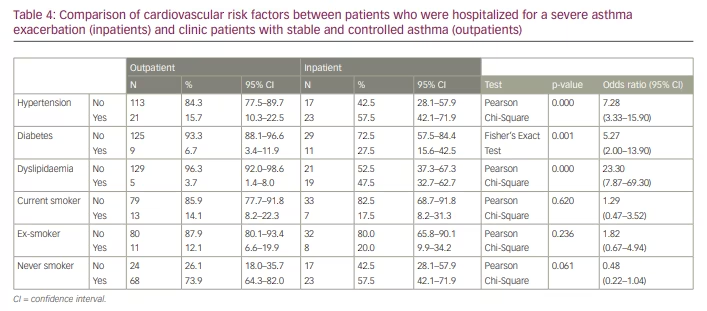
Of inpatients, 27.5% (95% CI 15.6–42.5%) had diabetes compared with only 6.7% (95% CI 3.4–11.9%, p=0.001) of outpatients (Table 4). Patients with asthma and diabetes were 5.27 times more likely to have a severe asthma exacerbation.
Almost half (47.5% (95% CI 32.7–62.7%) of inpatients had dyslipidaemia compared with only 3.7% (95% CI 1.4–8.0%, p<0.001) of outpatients (Table 4). Dyslipidaemia predisposed patients to a severe asthma exacerbation with an odds ratio of 23.30.
Asthma control assessment questionnaire
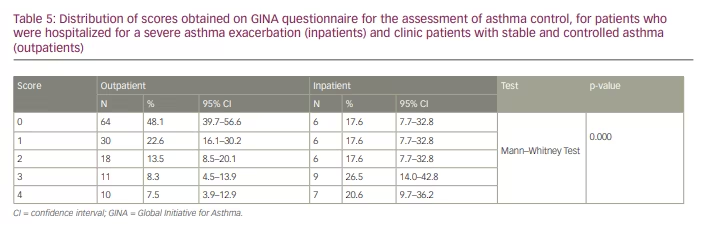
Asthma was better controlled among outpatients, when compared with inpatients. During the 4 weeks prior to the exacerbation, inpatients noted daytime asthma symptoms more than twice per week, nocturnal symptoms, need for reliever more than twice per week and/or activity limitation due to asthma. These elements, which show a low level of asthma control, were much less present among outpatients who had stable asthma. Around 70% of outpatients had a score of 0 or 1 on the GINA assessment questionnaire when it was performed during their visit to the clinic, whereas 47% of inpatients had a score of 3 or 4 prior to hospitalization, showing the absence of asthma control. The distribution of scores is shown in Table 5.
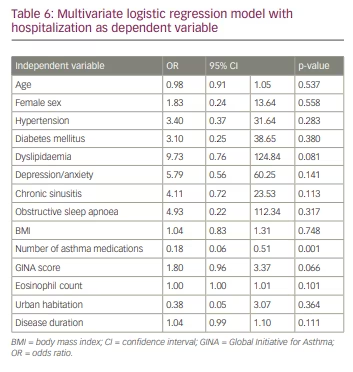
Using a multivariate logistic regression model, the number of medications was the main predictor of decreased hospitalization (multivariate-adjusted odds ratio=0.18 [95% CI 0.06–0.51]) for each incremental medication. Dyslipidaemia and GINA assessment of asthma control tended to be associated with an increased risk of hospitalization (Table 6).
Discussion
In our study, we found that epidemiological factors (female sex, higher BMI, rural habitat, older age and lower educational level), cardiovascular risk factors (hypertension, diabetes and dyslipidaemia) and asthma-related factors (obstructive sleep apnoea, chronic sinusitis, high eosinophil levels and poor asthma control), predisposed patients to severe asthma exacerbation requiring hospitalization.
Exacerbation is a defining feature of severe asthma.17 Patients with asthma are required to take their medication continuously to optimize their disease control. An exacerbation may occur, causing a drop in oxygen levels with inability to breathe, requiring the administration of short-acting bronchodilators and corticosteroids. Asthma exacerbations are treated with inhaled or nebulized short-acting β agonists, such as albuterol or levalbuterol, which can initially be used every 15–20 minutes for the first hour during acute asthma exacerbation.21 Adding ipratropium bromide, which is a short-acting muscarinic antagonist, helps reduce hospitalization rate.22 The administration of systemic corticosteroids is also recommended by GINA, which recommends systemic corticosteroids for a number of days (usually 5–7 days) in the treatment of severe exacerbations.20 Antibiotics are not always indicated.
The need for hospitalization varies, and requires an assessment of the exacerbation. Asthma exacerbation should be assessed continuously in two different stages: determination of the severity of the exacerbation by a static assessment, and evaluation of the treatment efficiency and patient’s response by frequent assessments.21 Hospitalization may be necessary following the exacerbation to monitor the patient’s vital signs and administer appropriate therapy until the patient recovers.
Age is an important factor for severe asthma exacerbation, which can be explained by many factors. Lung function declines with age.23 Moreover, older patients have more comorbidities than younger patients and may be less compliant with their treatment.24 Zein et al. found that, stratifying by age, the probability of severe asthma was higher in older patients compared with the young adult patients.25 The physiological decline of lung function and the comorbidities that are associated with advanced age make older patients more susceptible to developing severe exacerbations.24
Concerning obesity, fat deposits in the mediastinum and abdominal cavities reduce the compliance of the lungs and chest wall, and alter breathing pattern.26–29 Therefore, we suppose that obesity causes a restrictive lung disease that, when added to asthma, which is an obstructive lung disease, leads to more severe respiratory symptoms and a higher risk of exacerbation. Taylor et al. found that obese individuals with asthma had a higher frequency of persistent symptoms, a greater need for reliever medications or controller medication and had greater risk of severe persistent asthma, compared to individuals who were not obese.30 This is in concordance with our study in which we found that the mean BMI was 3.31 points higher among inpatients than outpatients.
Females are more prone to severe exacerbations.31 In our study, females with asthma were more likely to present with a severe exacerbation. Oestrogens may be implicated in asthma genesis and severity, since, after puberty, the prevalence and severity of asthma are greater among women, and are greatest in women with early menarche or multiple pregnancies.32 In a descriptive study of 425 severe exacerbations, Tattersfield et al. found that the main patient characteristic increasing the risk of severe exacerbation was female sex.31
Lower educational level is associated with uncontrolled asthma; it may be linked to patients having less awareness about their disease and the importance of regular and continuous treatment in disease control.33 As for rural habitat, rural-based patients may have a higher exposure to allergens that trigger exacerbations. A number of allergens are more prevalent in rural areas, including pollen, animal fur or feathers, dust mites and fuel combustion. However, urban exposure to air pollutants cannot be neglected. Moreover, Mutius et al. highlighted the association between microbe-rich living conditions found in rural areas (presence of farm animals, fermented food, unprocessed milk and other elements) and protection against childhood asthma.34 Hence, different factors in both rural and urban areas can have variable influence on asthma.
Higher levels of eosinophils correlated with a higher risk of severe exacerbations in our study. This is in concordance with other studies. A UK cohort study on a large number of patients found that patients with asthma who have a high eosinophil count (greater than 400 cells/μL) are at greater risk of severe exacerbations and have a lower level of asthma control.35 Eosinophils cause persistent inflammation, leading to constant damage of the airways.36 This leads to air trapping, aggravation of symptoms and frequent exacerbations.37 Therefore, eosinophil levels should be used to assess the risk of developing severe asthma exacerbations that may require hospitalization and close monitoring.
In our study, diabetes, hypertension and dyslipidaemia were predominant in patients who were hospitalized for a severe exacerbation. Moreover, they were independent risk factors leading to severe asthma exacerbation. These cardiovascular risk factors are probably related to obesity, metabolic syndrome and insulin resistance. A 4-year study in South Korea concluded that an increase in insulin resistance was associated with a decline in lung function in all patients.38 This can be explained by the increase in cytokines, such as tumour necrosis factor-α and interleukin-6, among individuals with insulin resistance; these cytokines play a role in lung inflammation and fibrosis.38–40 Hence, insulin resistance could be an additional factor accelerating lung damage among patients with asthma, who already have airway inflammation, leading to an increased exacerbation risk.
Obstructive sleep apnoea and chronic sinusitis are linked to a greater risk of severe exacerbations. In fact, partial or complete obstruction during sleep apnoea stimulates the vagal nerve, which can trigger an asthma attack and nocturnal asthma by stimulating muscarinic receptors located in central airways.41–44 Concerning chronic sinusitis, sino-nasal inflammation can aggravate lower airway disease, possibly by post-nasal drip, nasobronchial reflex or inflammation.45,46
Psychological factors are also implicated in severe exacerbations, as anxiety and depression were significantly more prevalent among hospitalized patients in our study. A study on severe asthma patients concluded that anxiety and depression aggravate asthma symptoms and increase the risk of hospital admission.47 In addition, Finnerty et al. found a high prevalence of depression and anxiety among patients needing more than three drugs and high doses of inhaled corticosteroids (ICS) to control their asthma, classified as steps 4 and 5 of asthma treatment in GINA guidelines.11,20 A cyclical relationship exists between these diseases: people who have uncontrolled asthma are more likely to suffer from emotional distress, which in turn leads to a lower adherence to treatment and healthcare consumption.48
Patients with asthma presenting with pulmonary bacterial infection (presence of fever, hyperleukocytosis, increased C-reactive protein blood levels, infiltrates or condensation on lung imaging) were excluded from our study. However, other infections (e.g. viral or bacterial rhinosinusitis, bronchitis) could not be eliminated. The factors identified in our study may influence host immunity, making patients more prone to infections and exacerbations. Mechanisms underlying the effects of different factors identified in our study on host immunity need to be investigated.
As multivariate analysis showed that the number of medications strongly correlated with the risk of exacerbation, and that medications decreased the risk of hospitalization in a multiplicative way, we conclude that the effect of the different factors identified in our study on asthma exacerbation may be modified and reversed by asthma medications.
Our study findings are concordant with other studies. An analysis of baseline data from the National Heart, Lung, and Blood Institute Severe Asthma Research Program 3 correlated high BMI, high blood eosinophils and chronic sinusitis with frequent asthma exacerbations.17 Another study in Japan implicated high BMI and severe asthma as factors predisposing patients to asthma exacerbation.19 This suggests that, despite different social and healthcare systems, similar factors are associated with asthma exacerbations and can be targeted on a global level.
Recommendations
Obesity has serious consequences on human health, including that of patients with asthma. To reduce exacerbation risk, patients with asthma and obesity should make lifestyle changes to reduce their weight. Medical and/or surgical treatment for obesity is necessary in case of failure of lifestyle changes. A restrictive syndrome probably caused by obesity exacerbates asthma and worsens lung function. Moreover, controlling blood pressure, diabetes, cholesterol levels and triglyceride levels is important, as they are linked to insulin resistance, which aggravates lung damage by causing persistent inflammation. Anxiety and depression can worsen asthma symptoms and can also be caused by asthma, hence a psychiatric consultation is important to assess and treat these diseases, to stop the vicious cycle between asthma and anxiety or depression. Obstructive sleep apnoea and chronic sinusitis, like all asthma comorbidities, should be screened and treated.
Another important element is patient education. Asthma patients should be well educated about their disease and the importance of adherence to treatment, which is prescribed according to the stage of the disease. Patients should be informed about the importance of visiting their physician at the clinic to assess asthma evolution and step up the treatment if necessary, to achieve a high level of disease control and avoid exacerbation.
One important point is that blood eosinophil level is a good marker of asthma severity and can be used by physicians to assess patients with an eosinophilic phenotype of asthma for follow-up, and evaluate exacerbation risk.
We identified several independent risk factors that are implicated in severe exacerbation of asthma. Many of these can be targeted by a healthy lifestyle (appropriate diet, exercise, reduction of tobacco and alcohol consumption, etc.) and/or medical therapy, non-invasive ventilation for obstructive sleep apnoea, and intranasal saline/corticosteroids/antibiotics for chronic sinusitis. Targeting these factors would contribute to a greater control of asthma symptoms and reduce the rate of exacerbation, thereby improving the quality of life of patients with asthma. More studies need to be done to further evaluate the impact of different factors on severe asthma exacerbations and prove the importance of targeting these factors to achieve optimal disease control and limit exacerbation risk.
Limitations
Our study has several limitations. First, we proved the presence of independent risk factors for severe asthma exacerbation using statistical tests, but causality between these factors and severe asthma exacerbation remains to be proven. Second, selection bias may have been caused by the patients being selected from one medical centre and one clinic in Beirut. Third, for some patients, some variables were missing, but we used appropriate statistical tests to get significant results.
Conclusion
Our study shows that several factors are implicated in asthma exacerbation among hospitalized patients, including epidemiological factors (i.e. female sex, higher BMI, rural habitat, older age and lower educational level), cardiovascular risk factors (i.e. hypertension, diabetes and dyslipidaemia) and other factors, such as obstructive sleep apnoea, chronic sinusitis, high eosinophil levels and poor asthma control. These factors should be evaluated and treated among patients with asthma to reduce the risk of symptoms worsening and severe asthma exacerbation. They can also be targeted on a global level to reduce disease burden and costs.