Alpha-1 antitrypsin (AAT) deficiency (AATD) is the second most common genetic disease to cause clinically significant lung disease, surpassed only by cystic fibrosis.1 AAT is a serine protease inhibitor primarily produced in hepatocytes, with a normal plasma level of 20–53 µM or 90–200 mg/dL and a half-life of 3–5 days.2 AAT is transported via the systemic circulation to the lung, where it is actively taken up by the lung endothelial cells and transferred to the epithelial cells and alveolar space.3 AATD results from inherited mutations within the SERPINA1 gene, with Pi*ZZ being the most common genotype associated with clinically significant disease, leading to low serum levels of the AAT antiprotease and protease/antiprotease imbalance. Qualitative defects of AAT result from acquired post-translational modifications, such as polymerization, oxidation and nitrosylation, and are associated with decreased and inefficient neutrophil elastase inhibition despite low or normal serum levels of the AAT protein.4,5
Alpha-1 antitrypsin lung disease
Ever since genetic testing has been recommended for all individuals with chronic obstructive pulmonary disease (COPD),6 AATD has been identified as the most common genetic risk factor for early emphysema in both never- and active-smokers.7 In fact, 3–10% of patients with emphysema-predominant COPD have abnormal AAT variants.1,8,9 The classic presentation of AATD is severe, panacinar, bilateral, lower lobe emphysema, airflow limitation and decreased diffusion capacity for carbon monoxide (DLCO).1
An imbalance between proteases, such as neutrophil elastase, and anti-proteases, including AAT, with subsequent degradation of elastic fibres, has been the main pathobiological mechanism implicated in the development of AATD-related emphysema.10 Degradation of elastin produces crosslinked elastin peptides containing desmosine and isodesmosine, which are excreted in the urine; levels of these peptides correlate with emphysema progression and treatment response.11,12 Uninhibited neutrophil elastase present in AATD results in increased fibrinogen degradation products in the serum, which can also be used as a biomarker for treatment response.12–13
Traditional markers of COPD severity, such as lung function decline and computed tomography (CT) measurements of lung density, require a certain level of lung injury to effectively diagnose and monitor the disease. Therefore, it has been suggested that plasma markers of elastin degradation (e.g. matrix metalloprotease-3 and -9), inflammation (e.g. C-reactive protein, ceruloplasmin) and repair (e.g. leptin, gelsolin, insulin-like growth factor binding proteins) may become alternatives to assess the severity of emphysema at baseline, and its progression.11,14,15 Inflammatory mediators in the bronchoalveolar lavage such as interleukins, cytokines (including tumour necrosis factor-α), eotaxin and growth factors, have been used to monitor treatment response.16,17 None of these biologic signals has, to date, been recommended by guidelines or regulatory authorities for clinical disease management.
While the association between AATD and early emphysema is well described in patients with the Pi*ZZ genotype, AATD’s association with airway diseases manifesting as bronchiolitis, bronchitis and bronchiectasis is less well characterized.18 Of these, only severe bronchiectasis is considered a ‘clinically relevant’ AATD manifestation, but only when involving more than one lobe and associated with symptoms of cough and sputum production.19 However, all airway structural changes, even when minimally symptomatic, may impact clinical outcomes in AATD lung disease, including health status17 and airflow limitation.20
Established therapies
Current data suggest that individuals with AATD diagnosed with COPD benefit as much from medical treatment with bronchodilators, inhaled corticosteroids, pulmonary rehabilitation, supplemental oxygen therapy, low-dose azithromycin, roflumilast and immunizations as those with cigarette smoke-induced COPD.21 Patients with AATD are considered poor candidates for lung volume reduction surgery (LVRS) due to homogeneous, predominantly lower lobe emphysema, which contrasts with the predominantly upper lobe localization of emphysema in patients with COPD who typically benefit from LVRS.22 Survival post-double-lung transplantation for end-stage lung disease is similar in patients with AATD and those with cigarette smoke-induced COPD, rendering lung transplantation a viable option.23
The concept of maintaining a ‘protective threshold’ of AAT serum levels above 11 µM (50 mg/dL),24 although somewhat controversial, has been the cornerstone of developing AAT replacement or restoration therapies for AATD. Augmentation therapy, which involves weekly intravenous infusion of purified human AAT aiming to achieve levels ≥11 µM, is the most widespread therapeutic approach for AATD.25 Augmentation therapy with 120 mg/kg every 2 weeks has also been studied as an alternative to weekly dosing, but serum AAT levels were shown to decrease below 11 µM 1–2 days prior to the next dose.26 As such, weekly augmentation therapy with AAT 60 mg/kg has become the standard of care for patients severely deficient in AAT, especially those with forced expiratory volume in one second (FEV1) ≤65% predicted, in whom we have the best evidence for significant clinical benefit.27
Tortorici et al., who found that clinical efficacy did not plateau as AAT exposure was increased, suggested that there may be a role for higher AAT doses.28 The SPARK trial (Safety and Pharmacokinetics of Alpha-1 Proteinase Inhibitor in Subjects with Alpha-1 Antitrypsin Deficiency; ClinicalTrials identifier: NCT01213043) in 2013 found that double-dose 120 mg/kg/week achieved a higher mean steady state serum concentration of 27.7 µM (versus 17.3 µM in the 60 mg/kg/week group) with a similar safety profile.29 A follow-up pilot study with eight patients with AATD was conducted in 2019, which found that double-dose therapy for 4 weeks significantly reduced serine protease activity in bronchoalveolar lavage fluid (BALF) and plasma, and reduced markers of elastin degradation (desmosine, isodesmosine) in BALF.16
Because the kinetics of augmentation therapy are well known, measurement of trough serum levels after 60 mg/kg weekly or 120 mg/kg/week doses are not recommended. The SPARTA trial (Efficacy and Safety of Alpha1-Proteinase Inhibitor (Human), Modified Process (Alpha-1 MP) in Subjects With Pulmonary Emphysema Due to Alpha1 Antitrypsin Deficiency (AATD); ClinicalTrials identifier: NCT01983241), an ongoing phase III clinical trial, will evaluate whether the progression of lung tissue loss is mitigated in patients with AATD lung disease receiving augmentation therapy at 120 mg/kg/week compared with 60 mg/kg/week.30 If this higher dose regimen shows additional benefit, it will not only change therapeutic approaches for patients severely deficient in AAT, but will also prompt consideration of augmentation therapy for intermediately deficient (Pi*MZ) individuals with lung disease, with circulating AAT levels above 11 µM. Of note, current guidelines advise against using augmentation therapy in patients with the Pi*MZ genotype, since most will not develop significant lung disease unless being exposed to cigarette smoking.31
In addition to measuring the rate of FEV1 decline, lung density quantified by CT, a more sensitive test, performs well in clinical trials to longitudinally estimate the efficacy of augmentation therapy in patients with AATD.32 Dirksen et al., 1999,33 Dirksen et al., 2009,34 and Chapman et al., 2015 (RAPID study; Zemaira in Subjects with Emphysema Due to Alpha1-Proteinase Inhibitor Deficiency; ClinicalTrials identifier: NCT00261833),35 are the three seminal randomized controlled trials that chose decline in lung density measured by CT as a primary or secondary outcome to study the efficacy of augmentation therapy.35 Similarly, a 2-year open-label extension trial (RAPID-OLE; Extension Study of Zemaira® i.v. Administration in Subjects With Emphysema Due to Alpha1-Proteinase Inhibitor Deficiency; ClinicalTrials identifier: NCT00670007) showed that, compared with 2 years of placebo, augmentation therapy slowed the rate of lung density loss.36 Given the irreversible nature of the loss of lung parenchyma (that comprises lung density), early treatment recommendations were reinforced.36 A post hoc analysis of the RAPID and RAPID-OLE trials compared the rate of lung density loss in augmentation therapy versus placebo, and projected a gain of 5.6 life-years, defined as the time to terminal respiratory failure, in the treatment group.35–37 Also, a retrospective analysis of the National Heart, Lung, and Blood Institute AATD Registry found that augmentation therapy was associated with improved survival in individuals receiving augmentation therapy at all 10% increments of FEV1 from 10% to 60%, further supporting early initiation of augmentation therapy.38 Interestingly, few studies have studied the role of augmentation therapy in AATD lung transplant recipients; however, post-transplantation augmentation therapy is still recommended by several experts.39
Multiple commercial preparations of AAT from pooled human plasma have been developed over the years, which have similar efficacy and safety profiles, but may differ in the convenience of infusion duration and setup. For example, formulations with a higher purity profile allow for a more concentrated product and thus a lower infusion volume and time.40 Liquid formulations (compared with the standard lyophilized formulation) make administration more convenient by eliminating the need for product reconstitution, reducing storage space needed given the lack of diluent packaging, and requiring less volume for infusion.41,42
Despite the potential survival benefits of augmentation therapy, indefinite weekly infusions come with significant financial, emotional and social burden on patients with AATD and their families. In fact, the estimated annual medical cost among US patients with AATD on augmentation therapy is $127,537 compared with $15,874 for those with AATD receiving usual COPD care without augmentation therapy, with 75.3% of the cost difference attributable to augmentation therapy.43 In a recent review, Chorostowska-Wynimko et al. advocated for patient-centric initiatives to improve the convenience of AAT therapy through extended-interval dosing, such as double-dose every other week during vacations, or self-infusion at home.44 In addition, Campos et al. showed that a health management programme, which includes a clinical coordinator and infusion and insurance specialists, reduced the rate of severe exacerbation episodes by 36.1% and adjusted total all-cause and respiratory-related costs by 11.4% and 10.6%, respectively.45 The COVID-19 pandemic has made it difficult for many patients to access the hospital for augmentation therapy and, unfortunately, home-based therapy is an alternative offered in very few countries. Annunziata et al. showed that 16 patients receiving home-based intravenous augmentation therapy had improved quality of life compared to patients without home-based therapy.46
The highly specialized care required for AAT infusions, the unproven efficacy in heterozygous patients with AATD and COPD, the inability to fully recover the lung function lost at the time of treatment initiation and the limited availability of human plasma for AAT purification has put pressure on the field to develop personalized therapies that are easier to administer in both early and advanced AATD lung disease and, if possible, target a specific SERPINA1 mutation.
Novel therapies
A new attempt at redefining therapy in AATD was to extend the half-life of human AAT with an engineered, recombinant, human AAT-Fc (fragment crystallizable region) fusion protein (INBRX-101; Inhibrx, La Jolla, CA, USA). When administered at 120 mg/kg every 3 weeks, it maintains the AAT serum level in the expected range, while its maximal anti-elastase activity is preserved. Its efficacy is currently unknown; an open-label phase I study was started in July 2019 and will enroll patients until December 2021 (ClinicalTrials.gov identifier NCT03815396).47
To address the occasional shortage of pooled human plasma for AAT purification, the field has turned towards finding alternative sources of AAT, from recombinant AAT product from plants to reprogramming progenitors/stem cells to secrete exogenous human AAT when delivered in the lung.48 In the era of regenerative medicine, we and others have shown that the adipose-derived mesenchymal stem cell (MSC)-secretome contains AAT with high anti-elastase in vitro activity and other proteins involved in protease/anti-protease balance.49,50 These findings position allogenic MSC as a novel therapeutic option for AATD patients.
Although augmentation therapy has traditionally relied on delivering AAT intravenously (since AAT produced in hepatocytes reaches the lungs through systemic circulation), inhaled AAT achieves much higher local levels of AAT in the airway epithelial lining.51,52 Inhaled AAT may be less expensive and more convenient for patients than intravenous formulations.53,54 However, the enthusiasm for its use was tempered by the findings of Stolk et al.; when 168 patients were randomized to receive twice-daily inhalations of 80 mg AAT solution or placebo for 50 weeks, the average yearly exacerbation rate in the inhaled AAT treatment group was similar to that of the placebo group, with more patients receiving inhaled AAT reporting treatment-related adverse events, prompting modifications of the nebulizer used.55 Other trials of inhaled AAT are ongoing (ClinicalTrials identifiers: NCT01217671, NCT02001688, NCT00161707, NCT01983241)56–59 that will report whether inhaled AAT is safe, well tolerated and clinically effective.60
Other approaches to ameliorate the damage to lung tissue caused by the protease/anti-protease imbalance in AATD are emerging. Hyaluronan or hyaluronic acid (HA), a long-chain polysaccharide that protects against elastin fibre breakdown, is a promising new therapy.61 Patients with AATD and COPD were found to have significantly decreased HA levels in lung tissue (13.5 ng/mg wet lung; n=9) compared with controls (21.7 ng/mg wet lung; n=5), with a direct correlation between HA levels and FEV1, DLCO and serum AAT levels.62 Cantor et al. conducted a 2-week phase IIa safety trial in 11 patients with COPD (eight receiving HA 0.01% aerosol twice a day for 14 days, and three receiving placebo) and did not observe significant adverse events.63 They followed this study with a phase II, 28-day randomized trial of twice-daily HA 0.03% in patients with AATD and COPD; the results, published in 2021, show a progressive reduction in free urine desmosine and isodesmosine in the HA group starting at day 14, which was maintained up to day 35.64
Following promising results in non-cystic fibrosis bronchiectasis, the reversible oral inhibitor of neutrophil elastase, alvelestat (Mereo BioPharma, London, UK), has made important strides as a novel therapy for AATD lung disease.65 It is currently under investigation in two phase II, placebo-controlled clinical trials, ASTRAEUS (A 12-week Study Treating Participants who have Alpha1-Antitrypsin-Related COPD with Alvelestat (MPH966) or Placebo; ClinicalTrials identifier: NCT03636347)66 and ATALANTa (Alvelestat (MPH966) for the Treatment of Alpha-1 Antitrypsin Deficiency; ClinicalTrials identifier: NCT03679598).67 Alvelestat is not the only oral protease inhibitor currently in clinical trials. PHP-303 (pH Pharma, Seongnam, South Korea) is a novel molecule being studied in phase II clinical trials primarily targeting AATD liver disease, with secondary endpoints including lung outcomes pertinent to AATD lung disease.
Another therapeutic approach is to correct and promote the proper folding of the mutant Z-AAT protein. VX-864 (Vertex Pharmaceuticals, Boston, MA, USA), an oral small molecule corrector, was studied in a phase II proof-of-concept study in patients with the Pi*ZZ genotype (ClinicalTrials identifier: NCT04474197),68 where it led to consistent increases in mean functional AAT levels of 2.2–2.3 µM from baseline without significant adverse events.69 However, the magnitude of VX-864’s effect as studied may not translate into substantial lung protective effects, as AAT plasma levels remained well below the accepted threshold for efficacious inhibition of neutrophil elastase.69
From the therapies targeting the liver, ARO-AAT (Arrowhead Pharmaceuticals, Pasadena, CA, USA) is a hepatocyte-targeted inhibitory ribonucleic acid (RNAi) designed to inhibit the expression of the mutated Z-allele of alpha-1 antitrypsin (Z-AAT) messenger RNA (mRNA) and subsequently reduce Z-AAT protein synthesis. Preliminary data in patients with the Pi*ZZ genotype showed significant improvement in liver parameters at week 24 and no detrimental or beneficial effect on AATD lung disease.70
Although the lung disease in patients with the Pi*ZZ or Pi*SZ genotypes progresses independent of their liver disease status, those with end-stage liver disease who are candidates for liver transplantation because of the severity of their liver dysfunction may also have a slower rate of lung function decline post-liver transplantation.71 This may be linked to normal AAT levels detected in patients soon after transplantation.72 Due to its risks, liver transplantation is not being considered for patients with AATD lung disease in the absence of severe liver dysfunction.71,72
Gene therapy offers the possibility for a one-time administration to provide therapeutic levels of AAT to protect against lung injury. Lungs are a promising organ for gene therapy, since the vector can be delivered directly via nebulization, pleural administration or intramuscular administration; however, challenges include the barrier posed by the mucus lining of the airway epithelium, which can trap vectors, and the relatively rapid renewal of the epithelial cells requiring repeated therapeutic administration.73 Adeno-associated viral (AAV) vectors, considered safer than other types of vectors because they do not integrate into the genome, were first used in AATD gene therapy.73 In a phase I clinical trial, recombinant AAV serotype 2 vector expressing AAT (rAAV2-AAT) was administered via intramuscular injection in 12 patients with AATD. The therapy yielded low levels of wild-type AAT, with the emergence of anti-rAAV2 antibodies.74 Interim results of a phase II trial using rAAV1-CB-hAAT intramuscular injection in nine patients with AATD indicated that the serum AAT levels were dose dependent, peaked at 30 days, but then decreased to 3–5% off the target concentration for at least 90 days after a single administration.75
One current focus of gene therapy revolves around intrapleural administration of vectors, which, in mice, generated higher lung and serum AAT levels than intramuscular administration.76 Following the emergence of AAV rhesus macaque-derived serotype 10 (AAVrh.10-AAT) as the most efficient vector,77 a phase I and II clinical trial assessed two different doses of intrapleural AAVrh.10-AAT (ADVANCE; Safety Dose Finding Study of ADVM-043 Gene Therapy to Treat Alpha-1 Antitrypsin (A1AT) Deficiency; ClinicalTrials identifier NCT02168686).78–80 However, an abstract published shortly after the end of the trial in 2018 showed that this approach did not lead to significant elevations in serum AAT.81
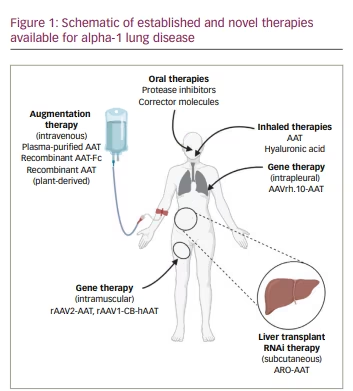
Discussion
Current AAT augmentation modalities for AATD lung disease offer significant advantages over regular COPD care, primarily by preserving lung function and CT lung density, especially in patients with the Pi*ZZ genotype and emphysema. Although it is challenging to study mortality benefits in this rare disease, post hoc and retrospective analyses of trials and registries have suggested an association between augmentation therapy and improved survival.82,83 Aside from the drawbacks of cost and the inconvenience of intravenous administration, the concept of augmenting AAT levels in deficient individuals could advance with novel sources of recombinant AAT either from plant or progenitor/stem cell sources. The real excitement comes from the progress being made with therapies targeting SERPINA1/AAT transcriptional and translational messaging, where gene therapy, RNAi, corrector molecules and novel oral protease inhibitors could alter AATD treatment in the next 3–5 years (Figure 1). Moreover, the new molecules will target AATD lung disease in a more personalized manner, which may address SERPINA1 mutations other than the Glu342Lys mutation (Z allele) and clinical phenotypes of AATD besides classical lower lobe emphysema.