There is a genuine interest on the part of researchers and the pharmaceutical industry in developing so-called ‘bifunctional’ drugs – that is, single molecules with two different primary pharmacological actions.1,2 In this context, single molecules that are capable of simultaneously inducing bronchodilation and anti-inflammatory activity are a highly intriguing possibility for treating a variety of obstructive pulmonary diseases as a longer-term alternative to the combination therapies that are currently the gold standard.3
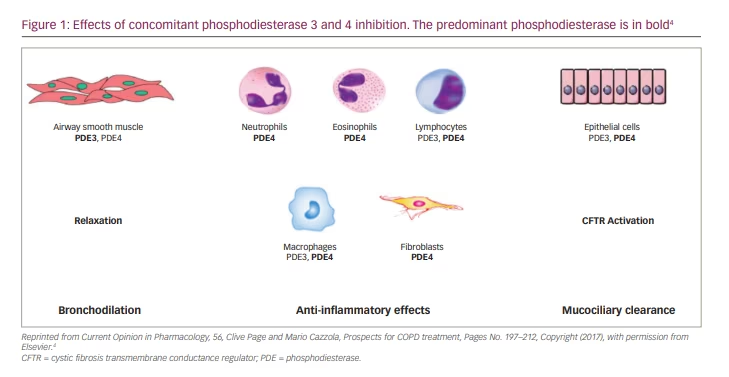
Concurrently, several classes of bifunctional molecules that can induce bronchodilation and anti-inflammatory activity have been investigated experimentally and in early clinical trials.1–3 One approach is to develop drugs that can simultaneously inhibit phosphodiesterase (PDE) 3 and PDE4.4 Drugs concurrently inhibiting PDE3 and PDE4 have been shown to provide additional or synergistic anti-inflammatory and bronchodilator benefits compared with drugs that inhibit either PDE3 or PDE4 alone.5 Furthermore, PDE3/4 inhibitors have been demonstrated to improve mucociliary clearance.6
PDE4 isoenzymes are a family of intracellular enzymes implicated in the pathophysiology of chronic airway disorders as they are expressed in most inflammatory cells, including neutrophils.7 Acute bronchodilator effects are not produced by PDE4 selective inhibition;8 rather, suppression of PDE3 – the PDE isoenzyme predominantly expressed in airway smooth muscle (ASMs) cells – results in bronchodilation.5 Furthermore, emerging data have suggested that inhibiting this PDE3 may elicit some anti-inflammatory effects,9 although this activity has not been observed by many other groups over the past 20 years.5,10
However, it has been proposed that the simultaneous inhibition of PDE3 and PDE4 may produce an additional benefit to inhibiting either enzyme alone due to the distinct physiological roles and major activity of these two PDE isoenzymes and because, often, both of these enzyme subtypes can be found in the same cell types (e.g. ASM, some inflammatory cells; Figure 1).2,4 Dual PDE3/4 inhibitors have therefore been developed to provide drugs with simultaneous bronchodilator and anti-inflammatory effects that may offer advantages over drugs inhibiting either enzyme subtype alone.1,2,5,7 However, the mechanisms that might underlie the apparent synergistic effect of dual PDE3/4 inhibition remain unclear.11 One hypothesis is that these two PDE isoenzymes govern different cyclic adenosine monophosphate (cAMP) pools intracellularly, with the PDE3 isoenzyme mainly found in the particulate cellular fraction and the PDE4 isoenzyme being primarily cytosolic.12
At present, ensifentrine, formerly known as RPL554 [9,10-dimethoxy-2(2,4,6-trimethylphenylimino)-3-(n-carbamoyl-2-aminoethyl)-3,4,6,7-tetrahydro-2H-pyrimido[6,1-a]isoquinolin-4-one],13 is the most advanced dual PDE3/4 inhibitor in development, having just completed a phase III clinical trial (A phase 3 clinical trial to evaluate the safety and efficacy of ensifentrine in patients with COPD; ClinicalTrials.gov identifier: NCT04535986) in patients with chronic obstructive pulmonary disease (COPD).3
Preclinical studies
Extensive preclinical studies in experimental animals have documented the remarkable ability of ensifentrine to induce ASM relaxation.13 Its ability to inhibit electrical field stimulation for at least 12 hours and to significantly lessen histamine-induced bronchoconstriction in anaesthetized guinea pigs for a lengthy period (5.5 hours) when administered by inhalation provided evidence of the drug’s potential to induce bronchodilation.13 Ensifentrine has also been shown to produce additional benefits on ASM relaxation when added to another class of bronchodilators, such as a muscarinic receptor antagonist or β2-adrenoceptor agonist.14
Of even greater relevance are data concerning the effect of ensifentrine on isolated human bronchial smooth muscle.15,16 These experiments have shown that ensifentrine not only relaxes human ASM directly but can also inhibit ASM tone, regardless of airway size, including small airways. Furthermore, ensifentrine acts synergistically with a muscarinic receptor antagonist (atropine or glycopyrronium), eliciting a remarkable increase in the duration of action of glycopyrronium. However, interestingly, there was no synergy when ensifentrine was combined with salbutamol, even when combined at isoeffective concentrations – only an additive effect.15
In addition, ensifentrine has been shown to inhibit the activation of several inflammatory cells in vitro13 to exhibit a strong anti-inflammatory effect in vivo in animals13 and to inhibit lipopolysaccharide-induced neutrophilia in sputum from human volunteers.17 It has also been reported that ensifentrine stimulates the cystic fibrosis transmembrane conductance regulator.18 This membrane protein functions as a channel for the chloride ion is involved in mucus hydration and is tightly regulated by the cAMP/protein kinase pathway, a signal pathway likely responsible for the noticeable increase in ciliary beating.18
The clinical development of ensifentrine in chronic obstructive pulmonary disease
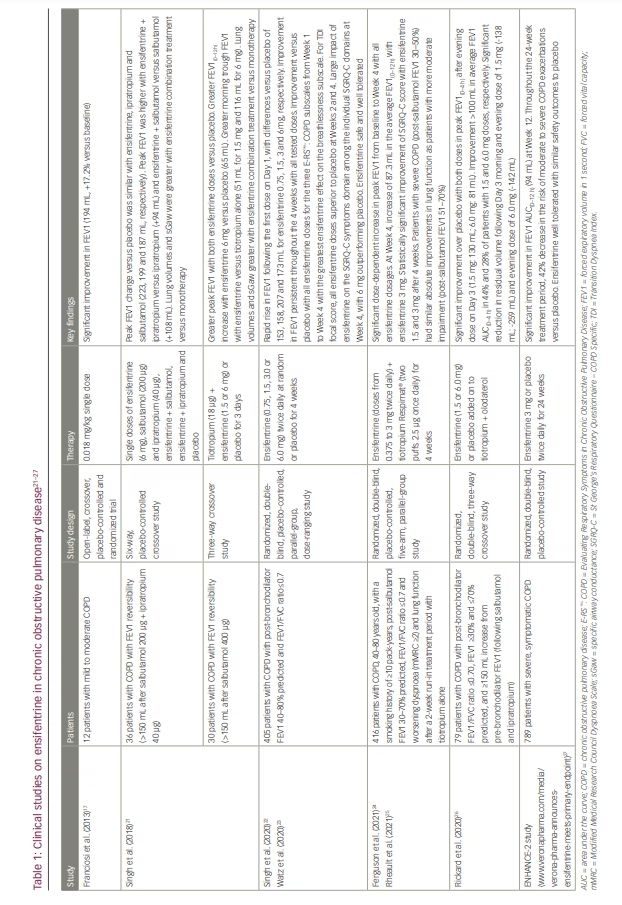
Ensifentrine was first created as a pH 3.2 solution in citrate/ phosphate-buffered saline for nebulization.19 Early pilot studies investigated this formulation for its safety and effectiveness in patients with asthma or mild to moderate COPD and in healthy volunteers (Table 1). In patients with COPD, ensifentrine (0.018 mg/kg) caused an increase in forced expiratory volume in 1 second (FEV1) from baseline that peaked at 194 mL (standard error [SE], 45.9; p<0.0001), which was an increase of 17.2% (SE, 5.2), comparable with the effect of salbutamol 200 µg (17.5% [SE, 11. 8]).17 Ensifentrine also significantly decreased the absolute number (but not the percentage) of neutrophils and the number of total cells, macrophages, eosinophils and lymphocytes recruited in the sputum 6 hours after a challenge of healthy participants with lipopolysaccharide.17
Subsequently, ensifentrine has been developed as a neutral pH phosphate-buffered suspension formulation for inhalation.19 When this formulation was administered to healthy male volunteers, ensifentrine had a significantly longer terminal half-life (10.2 versus 3.29 hours), a much longer plasma time to peak drug concentration (1.1 versus 0.17 hours) and a lower maximum plasma concentration (393 versus 1,816 pg/mL) than the prior formulation.19 A further study reported that the administration of ensifentrine in this new suspension formulation by nebulizer in single and ascending doses (1.5, 3, 6, 12, 24 mg) led to a significant, dose-dependent rise in FEV1 in healthy volunteers and patients with COPD at all dosages examined.20
The bronchodilator efficacy of ensifentrine (6 mg) given in combination with salbutamol (200 μg) or ipratropium bromide (40 μg) has also been assessed in patients with moderate-to-severe COPD (Table 1).21–27 Changes from baseline in peak FEV1 compared with placebo showed that ensifentrine on its own was an effective bronchodilator (223 mL; 95% confidence interval [CI]: 178–269; p<0.001), comparable with both salbutamol (187 mL; 95% CI: 142–232; p<0.001) and ipratropium (199 mL; 95% CI: 153–244; p<0.001). In addition, ensifentrine was associated with considerable and clinically significant additive bronchodilation when dosed with either salbutamol (peak FEV1 increased by 108 mL versus salbutamol alone; 95% CI: 63–153; p<0.0001) or ipratropium (94 mL; 95% CI: 49–139; p<0.0001). When given in addition to a single dose of tiotropium bromide (18 µg) for 3 days in patients with COPD, ensifentrine (1.5 or 6 mg) continued to provide quick (within 5 minutes) further bronchodilation, with a rise in peak FEV1 of 104 mL for the 1.5 mg dose and 127 mL for the 6 mg dose compared with tiotropium bromide alone (p=0.002 and p<0.0001, respectively), although the average FEV1(0–12 h) increase was greater with ensifentrine 6 mg only versus placebo (65 mL; p=0.0009). In addition, morning trough FEV1 rose compared with tiotropium bromide alone (by 51 mL, p=0.09, for 1.5 mg; by 116 mL, p=0.0009, for 6 mg). Ensifentrine significantly decreased residual volume and functional residual capacity more than salbutamol, ipratropium bromide or tiotropium bromide alone.
A phase IIb trial investigating the efficacy of ensifentrine randomized 405 patients with moderate to severe COPD recruited from 45 European locations to receive ensifentrine or placebo, with 375 patients (93%) completing the study (Table 1).22 Participants were given ensifentrine 0.75, 1.5, 3.0 or 6.0 mg or placebo twice daily for 4 weeks. All four ensifentrine groups experienced a rapid rise in FEV1 following the first dose on Day 1, with differences versus placebo of 153 (95% CI: 75–216), 158 (95% CI: 83–222), 207 (95% CI: 131–270) and 173 (95% CI: 69–210) mL for ensifentrine 0.75, 1.5, 3 and 6 mg, respectively (all p<0.001). Ensifentrine also caused a substantial improvement in FEV1 compared with placebo after the first week. Throughout the 4 weeks of therapy, all tested dosages continued to have a good bronchodilator effect, with differences compared with placebo falling between 139 and 200 mL, with a dose-dependent effect from 0.75 to 3 mg. The patients’ symptoms improved with all four ensifentrine doses compared with placebo, as evidenced by improvements in dyspnoea, chest symptoms, cough and sputum.
All four ensifentrine doses were superior to placebo for all three of the Evaluating Respiratory Symptoms (E-RS™: COPD) subscales (breathlessness, cough/sputum and chest symptoms) starting in Week 2 and showed progressive improvement versus placebo from Week 1 to Week 4 (Table 1).23 Ensifentrine showed the greatest effect on the breathlessness subscale. At Weeks 2 and 4, all ensifentrine dosages also outperformed placebo in the Transition Dyspnea Index focal score (p<0.05). Among the individual St George’s Respiratory Questionnaire – COPD Specific (SGRQ-C) domains (symptoms, activity and impacts), ensifentrine’s impact was largest on the symptoms domain at Week 4, with 6 mg outperforming placebo (p<0.05). Ensifentrine was well tolerated, and there were no reported safety concerns during the study.
Another phase IIb study examined the efficacy and safety of nebulized ensifentrine in doses ranging from 0.375 to 3 mg twice daily added to tiotropium bromide (two puffs 2.5 µg delivered via Spiriva® Respimat® once daily for 4 weeks) in 416 patients with COPD who presented with worsening dyspnoea and lung function after a 2-week run-in treatment period with tiotropium bromide alone (Table 1).24 From baseline to Week 4, peak FEV1 increased significantly and dose dependently with all ensifentrine dosages. The relative placebo-corrected differences for the 0.375, 0.75, 1.5 and 3 mg arms were 77.5 (95% CI: 4.8–150.1; p=0.037), 91.2 (95% CI: 18.0–164.3; p=0.015), 107.2 (95% CI: 34.4–180.0; p=0.004) and 124.2 (95% CI: 51.7–196.8; p<0.001) mL, respectively. At Week 4, the 3 mg dosage significantly increased the average FEV1(0–12 h) value by 87.3 mL (95% CI: 20.0–154.5; p=0.011). Furthermore, compared with tiotropium bromide alone, ensifentrine 1.5 and 3 mg improved quality of life (as measured by the SGRQ-C) after 4 weeks in a strong, clinically meaningful and statistically significant manner. This improvement was also accompanied by numerical improvements in COPD symptoms (as measured by the E-RS™: COPD and Transition Dyspnea Index).
The data from this trial were subsequently analysed by COPD severity at baseline.25 Patients were divided into two groups according to their pre-bronchodilator FEV1 baseline value: moderate COPD (51–70%) or severe COPD (30–50%). In the group with severe COPD, the 0.75 mg (102 mL; p=0.018), 1.5 mg (127 mL; p=0.003) and 3 mg (103 mL; p=0.019) ensifentrine arms showed statistically significant changes from baseline in peak FEV1(0–3 h) after 4 weeks. In the group with moderate COPD, the 3 mg arm produced a statistically significant improvement (120 mL; p=0.030). Although improvements did not achieve statistical significance, the placebo + tiotropium-corrected change from baseline average FEV1(0–12 h) revealed improvement in the 3 mg arm in both the severe and moderate groups (65 and 87 mL, respectively). The SGRQ-C total score for both subgroups, as measured by the placebo + tiotropium-corrected change from baseline, indicated significant numerical improvements in all arms.
In a further phase II trial, twice-daily nebulized ensifentrine (1.5 or 6.0 mg) added to a tiotropium bromide + olodaterol (5/5 μg) combination did not lead to statistically significant improvements in peak FEV1(0–4 h) on Day 3 compared with placebo (Table 1).26 However, peak FEV1 increased substantially over placebo following the evening administration of both doses on Day 3 (1.5 mg: 130 mL; p<0.001 6.0 mg: 81 mL; p=0.002), and 44% and 28% of patients improved their average FEV1 area under the curve at 0–4 hours by more than 100 mL with the 1.5 and 6.0 mg doses, respectively. On Day 3, the combination of ensifentrine, tiotropium bromide and olodaterol significantly reduced residual volume with the morning and evening doses of 1.5 mg ensifentrine (-138 mL, p=0.037, and -259 mL, p=0.002, respectively) and with the evening dose of 6.0 mg (-142 mL; p=0.036).
An assessment of the cardiovascular safety of nebulized ensifentrine in patients with moderate-to-severe COPD was undertaken by examining data from the two 4-week phase IIb trials.22,24 No appreciable effect of ensifentrine on vital signs or cardiac repolarization was seen.28 There were also no effects on morphology, rhythm disruptions or other electrocardiographic parameters, possibly because the drug levels in plasma are very low after inhalation.
Regulatory trials of ensifentrine
The top-line phase III ENHANCE-2 study, which investigated nebulized ensifentrine for the maintenance treatment of COPD, fulfilled its primary and secondary objectives, according to an announcement from Verona Pharma on 9 August 2022 (www.veronapharma.com/media/verona-pharma-announces-ensifentrine-meets-primary-endpoint). The study results have not yet been published in full or even presented at a congress as an abstract; the information available can be found in the press release from Verona Pharma. This study investigated the effect of ensifentrine in a large number of patients with COPD (n=789), who were randomly assigned to a 3 mg nebulized dosage of ensifentrine or a nebulized placebo twice daily for 24 weeks. However, around 52% of the participants also received background COPD medication with a long-acting muscarinic antagonist (LAMA) or long-acting β-agonist (LABA). In addition, 15% of all individuals received an inhaled corticosteroid (ICS) and LAMA or LABA simultaneously. With a placebo-corrected effect, ensifentrine resulted in significant bronchodilation; the average FEV1 area under the curve at 0–12 hours post-dose change was 94 mL at Week 12 (p<0.0001; Table 1). Furthermore, throughout the 24-week treatment period, ensifentrine-treated patients showed a 42% decrease in the risk of moderate-to-severe COPD exacerbations compared with placebo-treated participants (p=0.0109). Ensifentrine was well tolerated and had similar safety outcomes to placebo. According to Verona Pharma, additional data from the ENHANCE-2 trial will be presented at upcoming scientific conferences.
Another phase III study (ENHANCE-1), which is randomizing patients with COPD to 3 mg nebulized ensifentrine or nebulized placebo twice daily for 24 or 48 weeks, is on-going and will evaluate the longer-term safety and efficacy of the drug (A phase 3 clinical trial to evaluate the safety and efficacy of ensifentrine in patients with COPD; ClinicalTrials.gov identifier: NCT04535986).29
To confirm the anti-inflammatory effect of ensifentrine, a single-centre, randomized, double-blind, placebo-controlled, crossover phase II study is randomizing patients with COPD to receive either nebulized ensifentrine (3 mg) or placebo twice daily for two 8-week treatment periods (Effect of ensifentrine on sputum markers of inflammation in COPD; ClinicalTrials.gov identifier: NCT05270525).30 This trial is evaluating the effect of ensifentrine on sputum inflammation markers in COPD at Week 8: per cent change from baseline in acetylated proline-glycine-proline; absolute and per cent change from baseline in neutrophils, eosinophils, basophils, macrophages, lymphocytes and total cells; and per cent change from baseline in cytokines, proteases and other markers of inflammation.
Perspectives
Ensifentrine continues to show promise as a novel drug for treating patients with COPD, and the ENHANCE-2 study, even with the incomplete information available at the time, confirms the ability of this PDE3/4 inhibitor to induce significant bronchodilation and reports an interesting effect on the risk of exacerbation. However, as many patients in that study were on LAMA, LABA and even ICS background treatment, it will be necessary to further analyse the data and to understand how ensifentrine will be positioned, given there are still no formal comparisons between ensifentrine administered as chronic monotherapy with dual bronchodilation and the increasingly gold-standard triple therapy.
Further analysis of various subgroups will be necessary to try to understand whether ensifentrine will be able to replace or reduce therapy with the currently available classes of bronchodilators and/or whether it may be a valuable add-on therapy to the current standard of care to optimize bronchodilation further. In addition, it would be useful to know whether ensifentrine causes bronchodilation that persists beyond 12 hours during regular treatment and whether there is rebound bronchoconstriction when it is discontinued.
The results of the clinical studies so far strongly support the possibility of combining ensifentrine with a muscarinic receptor antagonist, as this combination has been reported to induce a substantial further increase in bronchodilation compared with the muscarinic receptor antagonist alone.21,24 In contrast, there is only weak evidence favouring the combination of ensifentrine with a β2-agonist.21
The lack of solid evidence to support the combination of ensifentrine with a β2-agonist has a rational pharmacological explanation. Two separate drugs can only interact synergistically when they operate on different pharmacological pathways.31 Ensifentrine acts as a dual inhibitor of PDE3/4, downstream effector proteins of the sympathetic signalling pathway triggered by β2-adrenoceptors.32 As a result, it raises cAMP levels. Ensifentrine and LAMAs, in contrast, work on different signal transduction pathways that ultimately combine to produce the ASM bronchorelaxant effect.16,31
It will be necessary to compare the combination of ensifentrine with a LAMA or a LABA to the well-established LAMA/LABA fixed-dose combinations that are currently on the market to determine whether it is useful and whether it can be considered a potential substitute for dual bronchodilation. Currently, the only available evidence is that many patients derive some benefit when ensifentrine is added to tiotropium bromide/olodaterol.28
The reduction in the risk of exacerbations that emerged in the ENHANCE-2 study is very intriguing. However, even here, it will have to be clarified whether that was the result of the addition of ensifentrine or the expected outcome of established treatment with long-acting bronchodilators and ICS. In addition, it needs to be understood whether adding ensifentrine reduced the exacerbation risk in patients with low blood eosinophils, who are known to respond poorly to corticosteroids.33 If this is the case, it will be essential to clarify the anti-inflammatory profile of ensifentrine. The results of the aforementioned phase II study (ClinicalTrials.gov identifier: NCT05270525) may be informative in this respect.30 If ensifentrine can reduce inflammation in patients with COPD, this would make this drug unique for its bifunctional properties. Even so, further work will still be needed to establish ensifentrine’s position compared with existing anti-inflammatory drugs, corticosteroids, xanthines and the orally active PDE4 inhibitor roflumilast.