Globally, the coronavirus disease 2019 (COVID-19) pandemic, caused by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), has infected in excess of 55 million people and is associated with over 1.3 million deaths to date (European Centre for Disease Prevention and Control data).1 COVID-19 is placing significant strain on all aspects of routine healthcare and has raised doubts over which management strategies should be maintained and which should be postponed. For patients with asthma and allergies, allergists are faced with finding the balance between the needs of the individual patient and the demands of public health.2 Understanding the differences between allergy and COVID-19 symptoms, and how to support patients with efficacious treatment is key to preserving favorable outcomes in a healthcare system facing myriad challenges.2,3
Q. What early signs of COVID-19 infection may differentiate the disease from respiratory allergy?
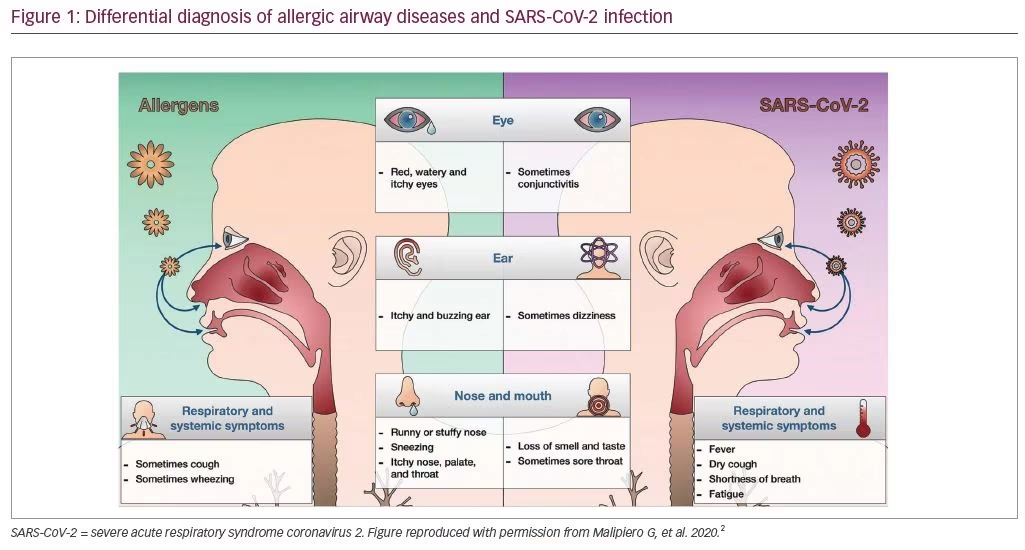
There are several key factors that can inform a differential diagnosis between allergic airway disease and COVID-19. The typical symptoms of allergy—itchy eyes, runny or stuffy nose, sneezing and wheeze—are not associated with COVID-19. Loss of smell, sudden loss of taste, fever, shortness of breath and fatigue are particularly important diagnostic criteria for COVID-19 (Figure 1).2
It appears that the risk of infection by COVID-19 may be reduced in patients with respiratory allergy. A key observation to support this is that COVID-19 uses angiotensin-converting enzyme 2 (ACE2) as its receptor. In vitro models have shown that ACE2 is downregulated in the nasal and bronchial epithelia by the cytokine interleukin (IL)-13.3 IL-13 is deeply involved in the allergic process and its expression increases in patients with allergic challenge. Therefore, patients with respiratory allergy are likely to be downregulating the receptors for the COVID-19 virus and susceptibility appears to be reduced.
Q. Are patients with allergies or asthma protected from COVID-19?
Early data from China suggested that patients with severe asthma were less likely to be infected by COVID-19.3 This position is supported by emerging data from Italy, where the COVID-19 infection rate in 1,504 patients with severe asthma was 1.73%.4 While there remains a great deal more to be investigated, it appears that cross‐regulation between allergic and interferon‐mediated immune responses in severe asthma may result in mild COVID-19 severity, even with a high viral load.5
Q. What are the current recommendations of the EAACI’s position statement regarding the use of allergen-specific immunotherapy?
The American Academy of Allergy, Asthma and Immunology (AAAAI) has produced a committee statement that discourages home administration of immunotherapy (and does not mention sublingual immunotherapy [SLIT]);6 however, this may be based on the practice of using just injection immunotherapy. This led to the publication of updated advice for the administration of allergen-specific immunotherapy in Italy, based on clinical experience.7 There are benefits to using digital medicine services—phone, video and email consultations—especially in patients with well-controlled, mild-to-moderate disease. There was success with the home delivery of biologics to allow self-administration, with support for the identification of near-home clinics for on-site administration as needed. Ongoing education for physicians and patients has been made available online.
The advantages of SLIT in allowing uninterrupted treatment have also become very apparent. Home delivery from the pharmacy means that patients do not have to use public transport or wait in clinics, and there have been no issues with administration with tablets, compared with injections.7
These findings were reflected in the Allergic Rhinitis and its Impact on Asthma and European Academy of Allergy and Clinical Immunology‘s position statement (ARIA-EAACI).8 The recommendations for Europe state that allergen immunotherapy can be continued as normal for patients without a history of COVID-19 exposure, without contact with carriers in the past 14 days, and with no symptoms of infection. The benefits of SLIT were reiterated for home administration and lowering risk of COVID-19 infection in the community.7
Q. What other advice has been given to allergy service staff?
Digital medicine is key to limiting exposure while retaining contact. Regular treatment should be continued for asthma and rhino-conjunctivitis. Patients should be provided with action plans for their conditions, and where possible, self-monitoring and reporting should be performed digitally. It is suggested that non-urgent pulmonary function tests are postponed.2,7 These approaches support the provision of ongoing management and treatment in allergic disease and asthma. As the COVID-19 situation evolves, digital medicine will continue to be a cornerstone of management—and this is likely to extend into the post-COVID-19 landscape.
Giorgio Walter Canonica
Professor Giorgio Walter Canonica is Professor of Respiratory Medicine at Humanitas University and Director of the Personalized Medicine Asthma and Allergy Centre at the Humanitas Research Hospital IRCCS, Milan, Italy. Professor Canonica initially specialised in pulmonary diseases after having received his medical degree from the University of Genoa, Italy; he then specialised in allergy and clinical immunology at Florence University, Florence, Italy. He subsequently conducted clinical immunology and allergology research in several European universities and institutes, in addition to 2 years at the Medical University of South Carolina, Charleston, SC, USA. Since returning to the University of Genoa in 1995, Professor Canonica has held a number of academic positions, including Director of the Speciality School of Allergy and Clinical Immunology, Director of the Specialty School of Pulmonary Diseases and Chairman of the Allergy and Respiratory Diseases Clinic. He is a board member of the Allergic Rhinitis and its Impact on Asthma (ARIA) guidelines committee, as well as board member of the Collegium Internationale Allergologicum (CIA), Vice President of the Respiratory Effectiveness Group (REG), Chair of the European Academy of Allergy and Clinical Immunology (EAACI) Methodology Committee, international advocate for the Global Initiative for Asthma (GINA) and a steering committee member of the Severe Asthma Network Italy (SANI) Registry. He is a fellow of the European Respiratory Society (ERS), the American College of Allergy, Asthma and Immunology (ACAAI), the American Academy of Allergy, Asthma and Immunology (AAAAI) and the EAACI. He has authored more than 750 peer-reviewed papers and has been cited over 60,000 times.