Lung, pulmonary, or broncholpulmonary sequestration is a congenital disorder in which tissue that develops into lung parenchyma is not attached to the bronchial system.1 Blood supply originates from the aorta or various peripheral arteries.1,2 It is also referred to as cystic lung disease. The intralobar type accounts for approximately 75% of cases; extralobar pulmonary sequestration accounts for 25% of cases, is located extrapulmonarily and forms its own visceral pleura.3,4 The majority of these lesions are diagnosed in children; intrauterine diagnosis using prenatal ultrasound and magnet resonance imaging are available.5 Intrauterine interventional procedures are an exciting new therapeutic option.6 Such lesions may completely regress during pregnancy. If they persist and are symptomatic, surgical removal is indicated. In adults, incidental detection of such lesions on computed tomography (CT) scan is common and patients may not have any symptoms. If symptomatic, pulmonary sequestration presents with chronic pulmonary infections, chronic dry cough, or hemoptysis.7,8 Treatment in adults is controversial and depends mainly on symptoms, and may include percutaneous embolization of the feeding artery, thoracoscopic, or open surgery.9–12 However, in many cases, no treatment is required,13 especially in patients of advanced age.14 Diagnosis of this disorder beyond the age of 60 years is extremely rare.13,14
Case report
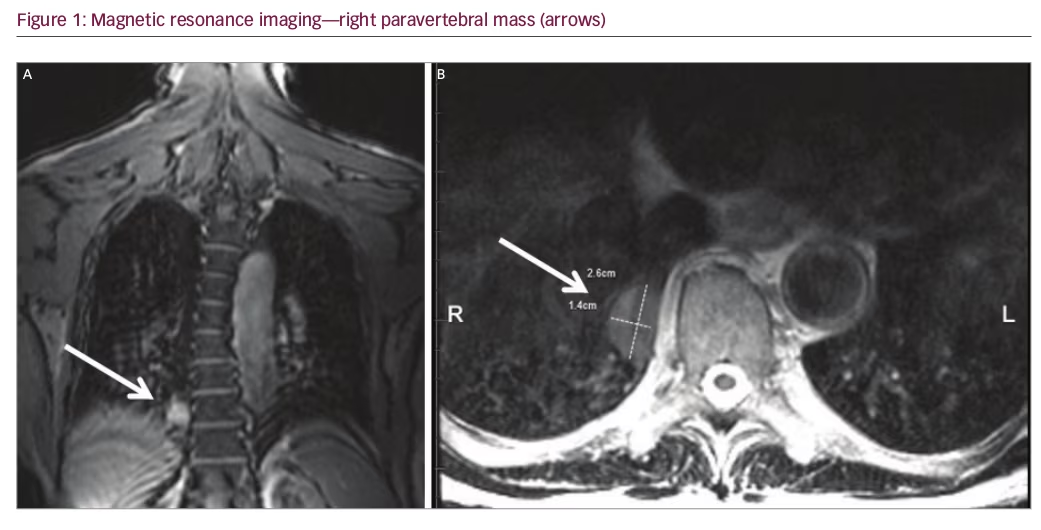
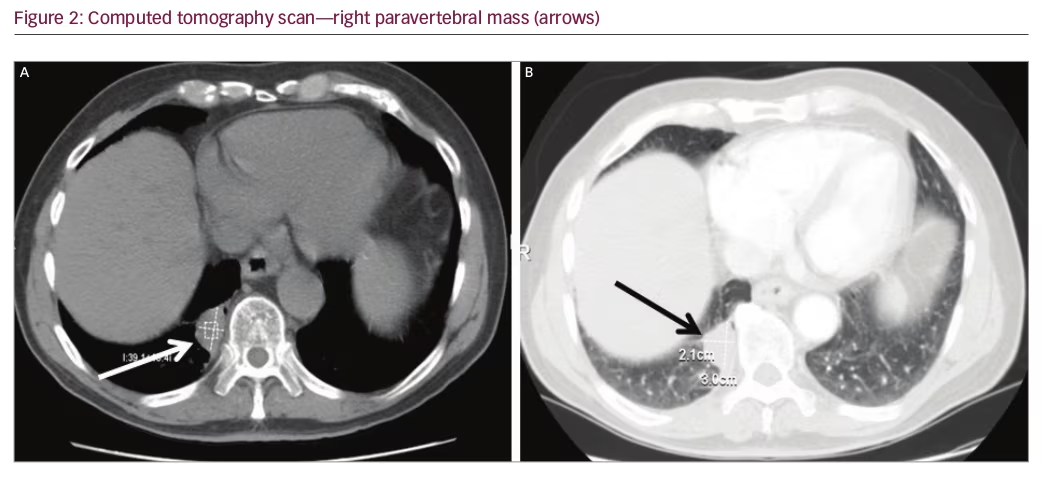
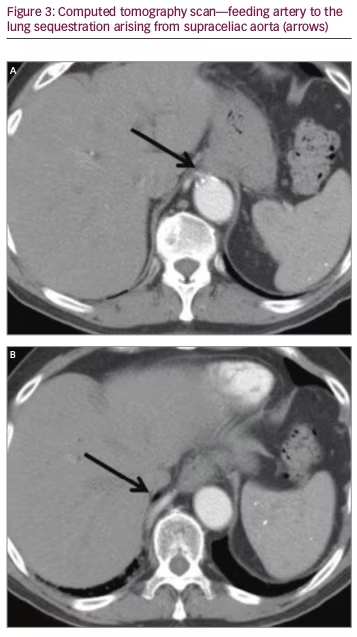
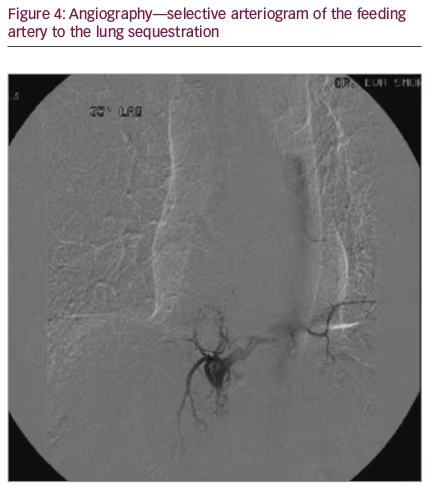
An 80-year-old male underwent a cardiac CT scan showing a 1.9 x 2.3 cm lesion in the right posterior mediastinum. The patient had no cough, chest pain, or other symptoms. A positron emission tomography (PET) scan was subsequently performed and the lesion was negative for radionuclide uptake. Magnetic resonance imaging (MRI) of the thoracic spine two years later showed again the stable mass (Figure 1A and 1B). He remained symptom-free for three years, after which he developed chronic cough. A contrasted CT scan was done and the mass at the lung base had not shown any growth (Figure 2A and 2B). The patient was referred to surgery and it was decided that a percutaneous CT-guided biopsy should be performed. On further review of the CT scan, however, a feeding artery from the intra-abdominal aorta was seen (Figure 3A and 3B) and it was then suspected that the lesion could be a pulmonary sequestration. An angiogram was performed and, indeed, the feeding blood vessel from the supraceliac aorta could be visualized, confirming the diagnosis (Figure 4). The lesion drained into the pulmonary venous system. No biopsy and no additional treatments were done and the patient was assured that this was a benign lesion and that his cough should be symptomatically treated. Initially, he did well, however, a year later he developed pneumonia, which was successfully treated with antibiotics. He is alive and well seven years after initial detection of the lesion.
Discussion
We report on an octogenarian male who presented with chronic cough and was found to have a pulmonary nodule. Differential diagnoses of such lesions in the elderly include benign and malignant tumors, infections, and malformations such as pulmonary sequestration.3 Primary lung cancer and metastases of various cancers and a variety of benign lung lesions such as neurogenic tumors need to be considered; PET/CT scan frequently helps in establishing diagnosis.15 It should be noted that this type of malformation is diagnosed extremely rarely in individuals older than 60 years.13,14 Montjoy et al. reported three adult patients with pulmonary sequestration; they were aged 57 to 62 years,8 and the oldest patient in the Gompelmann series from Germany was 59 years old.7 In a large series from Florida including 29 patients with pulmonary sequestration published by Tashtoush et al., almost two thirds of individuals were adults, with the oldest being 70 years old.11 The authors emphasized that many had a delay in diagnosis, as other more common pulmonary conditions were initially considered. Almost all patients in these series underwent surgical treatment.7,8 Diagnosis of pulmonary sequestration in adults may be difficult both on chest X-ray and uncontrasted CT scan. During the arterial phase of the contrasted CT scan, the feeding vessel, which commonly originates from the thoracic or supraceliac abdominal aorta may become visible, allowing accurate diagnosis.2,8,11 In our case we opted for aortic angiography. Huang et al. reported on the use of Doppler sonography in the diagnosis of pulmonary sequestration in another octogenarian patient, however, they subsequently confirmed findings on CT angiography.13 This patient also did not require intervention as he was not very symptomatic.
Our patient was not very symptomatic and did not desire any interventions given his advanced age. Many patients with smaller lesions do not require special treatment. If the lesion is symptomatic, causing recurrent infections, percutaneous embolization through the feeding vessel is an option. Embolization has also been performed in case of massive hemoptysis associated with pulmonary sequestration, as bleeding from these lesions may be fatal.9,10,16
To summarize, contrast-enhanced chest CT scan suggested pulmonary sequestration in this elderly gentleman and diagnosis was confirmed by identifying the feeding vessel with angiography. As the lesion was benign and the patient had only minimal symptoms, no additional interventions were made.