Tube thoracostomy (chest tube placement) is a common procedure used in pathologic conditions to drain the pleural space of air (pneumothorax), blood (hemothorax), serum (pleural effusion), lymph (chylothorax), pus (empyema), or a combination of these.1 In these instances, when an effusion or pneumothorax is nonloculated, a properly placed chest tube typically provides drainage and allows the lung to re-expand. However, once a chest tube is placed and drainage is inadequate, there are no recommended maneuvers for changing the tube’s position to enhance drainage of fluid or air. In addition, for multiloculated parapneumonic effusions, there are no prospective randomized trials to guide clinicians on the optimal treatment for an individual case. Instead, various strategies are available: additional tube placement, the use of intrapleural enzyme treatment,2 medical thoracoscopy, or video-assisted thoracoscopic surgery (VATS).3–5
In addition to limitations in positioning a chest tube after placement, there are also limitations on additional diagnostic procedures, such as pleural biopsies, which can be performed through the tube. Such biopsies may be valuable when previous diagnostic thoracenteses have been negative. In the setting of malignancy, pleural fluid cytology can be negative, with only an overall sensitivity of approximately 60 %.6,7 For tuberculous pleural effusions, visualizing acid fast bacilli has a low diagnostic yield of only 5–10 %8,9 and culture from pleural fluid only has a sensitivity of 50 % and may take up to 8 weeks for results.10,11
Unfortunately, once a standard chest tube is placed, it is not properly equipped to accommodate an endoscopic device through its lumen to take a pleural biopsy, so a separate invasive procedure such as closed-needle pleural biopsy (with or without ultrasound guidance) or thoracoscopy may be needed.
Previously, modifications have been proposed using standard bronchoscopy and tube thoracostomy to examine the pleural space.12–14 The ability to navigate the pleura and take biopsies was limited as there was a lack of scope mobility due to the fixed position of the chest tube and the lack of proximal rigidity inherent to a flexible bronchoscope, which led to reduced dexterity in the distal part of the scope.15
To overcome these disadvantages, a novel chest tube with a repositioning stylet has been developed (Cook Inc., Bloomington, IN, US). A repositioning stylet enables accurate post-placement repositioning of the device, improving the ability to drain a loculated fluid collection or air within the pleural space. In addition, a standard flexible bronchoscope can be introduced through the tube to allow for inspection and biopsy of all pleural surfaces. The stylet can be utilized to accurately direct the chest tube apically, medially, or inferiorly toward the diaphragm to allow for subsequent pleural intervention in those areas. In addition, an outer barrier sheath covering the steerable chest tube prevents contact of the access device and allows the tube to be advanced or retracted at various depths in the subject’s thorax. These two novel additions require minimal steering of the flexible bronchoscope when attempting to address different locations for biopsy.
We undertook this study primarily to assess the ability of a steerable chest tube (percutaneously or surgically placed) to change positions within the pleural space and allow for drainage of fluid. Second, we assessed the tube’s capacity for insertion of a standard flexible bronchoscope to visualize the pleural space and perform biopsies.
Materials and Methods
This is a single center animal study using a porcine subject to demonstrate proof of principle for a steerable chest tube in navigating, evaluating, and draining the pleural space. All procedures were performed under the guidance of an approved Institutional Animal Care and Use (IACUC) protocol.
The specific aims of this study are the following: (1) to demonstrate maneuverability for access and visualization using a steerable chest tube in a porcine pleural space; (2) to assess the steerable chest tube’s ability to drain fluid from the pleural space; (3) to demonstrate and assess the usefulness of the steerable chest tube for locating and biopsying implanted markers on the parietal pleural surface.
The steerable chest tube is a single-use, pleural access device that consists of a DEHP (di-2-ethylhexyl phthalate)-free, multistiff drainage catheter, smooth tapered inner obturator (for Seldinger method), and a curved repositioning stylet (see Figures 1–3). The stylet and catheter are available in one size, but are packaged in various procedural kits to allow for Seldinger or surgical placement. The total length of the steerable chest tube is 35 cm, outer diameter 28 French, and the inner diameter 19 French. An adjustable push clamp allows for locking of the tube at various desired depths, and
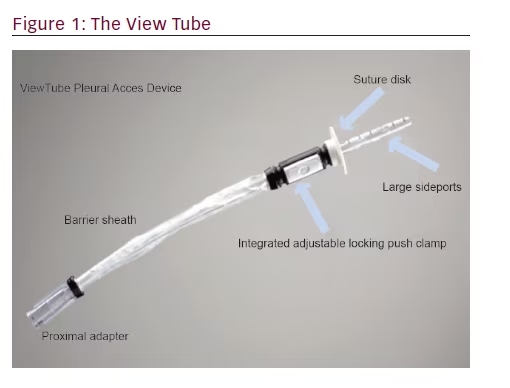

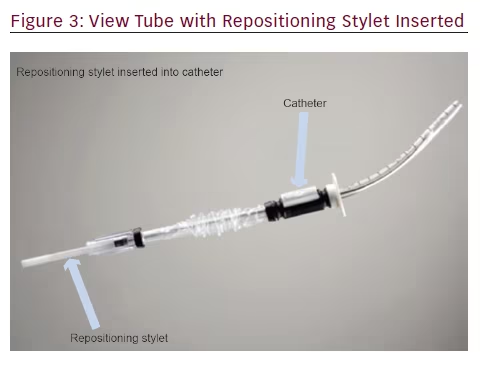
a clear outer barrier sheath allows for depth adjustment and positioning within the pleural space. In this manner, the pleural space can be accessed through insertion of a sterilized flexible bronchoscope.
One porcine subject was used for the study and was placed on mechanical ventilation under general anesthesia. Prior to the procedure, animal laboratory technicians created a single hydropneumothorax through a percutaneously placed pleural catheter (not the steerable chest tube), and three metal fiducial markers (Cook Triclip Endoscopic Clipping Devices, Bloomington, IN, US) were implanted endoscopically through the working channel of a bronchoscope. The pulmonologists were blinded to the placement of these markers. The markers were positioned on the apical, middle, and basilar regions of the parietal pleura, and the metal end of each marker was exposed to the inside of the pleural space. These markers served as biopsy targets when using the steerable chest tube system.
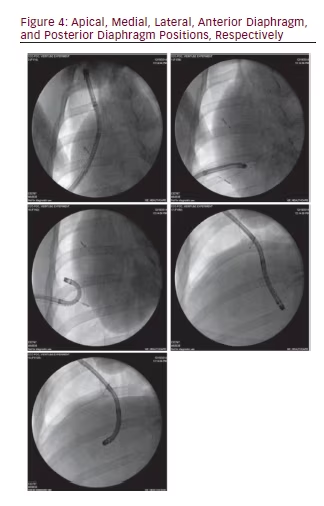
A single investigator blinded to the location of the fiducials, placed the steerable chest tube in the fifth intercostal space in the mid-axillary line using a modified Seldinger technique and it was sewn into place at the integrated plastic suturing disk. An area of chest wall was chosen that was not previously violated by any other invasive procedure. Following steerable chest tube placement, a standard flexible bronchoscope (BF-160, 5.2 mm outer diameter and 2 mm working channel, Olympus, Tokyo, Japan) was inserted through the steerable chest tube and positioned in five pleural locations (apical, medial, lateral, and along anterior and posterior diaphragm). After reaching each position independently with endoscopic views alone, fluoroscopy was used to confirm that the bronchoscope was in the desired location. Fluoroscopy images of the bronchoscope tip in each of five positions were recorded along with an endoscopic image of the location. When changing the bronchoscope’s orientation from toward the apex to toward the diaphragm, the ability to redirect the chest tube after initial steerable chest tube placement was also recorded as a yes/no variable.
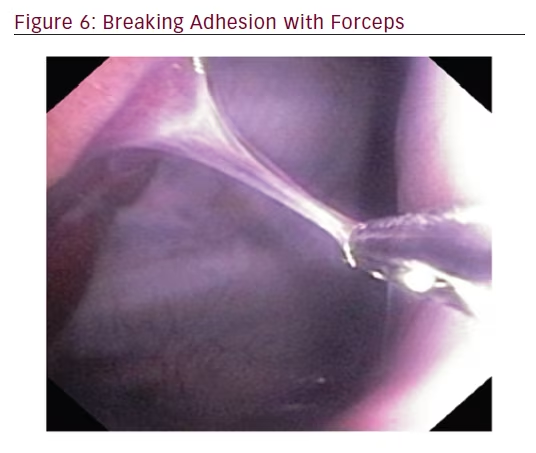
Following insertion of the steerable chest tube, the flexible bronchoscope was placed through the working channel of the steerable chest tube and attempts were made to locate the targets with the bronchoscope. When the target was visualized, flexible forceps were inserted through the working channel of the bronchoscope to close down on the metal end of each fiducial marker. Target localization and success of forceps closure on the fiducial marker was recorded as a yes/no variable. In addition, a naturally occurring adhesion was localized and disrupted with the flexible forceps.
Results
The placement of the steerable chest tube was accomplished on first attempt (by modified Seldinger and in a different location by surgical blunt-dissection) by a pulmonologist with no prior experience with the steerable chest tube and it provided access to the pleural space. Placement of the steerable chest tube within the pleural space was confirmed bronchoscopically, and maneuverability was facilitated by the redirecting stylet. This provided the user with the ability to direct and reposition within the pleural space. The following positions were reached and confirmed by all three pulmonologists: apical, medial, lateral, anterior diaphragm, and posterior diaphragm (see Figure 4).
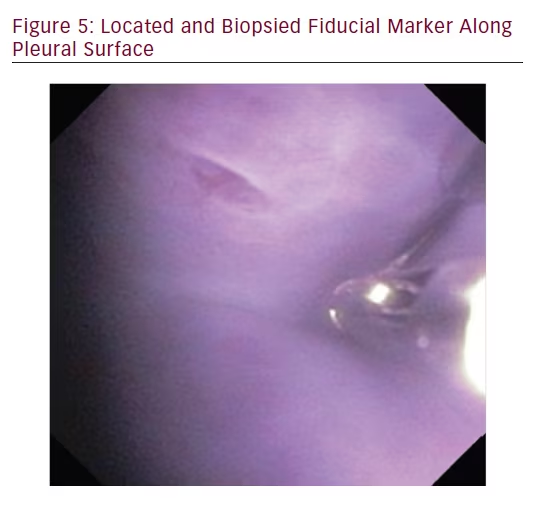
In order to locate and biopsy the metal fiducials placed prior to the procedure, the bronchoscope was passed through the steerable chest tube. All three pulmonologists were able to successfully locate and biopsy each fiducial via flexible forceps inserted through the working channel of the bronchoscope. The steerable chest tube also allowed the user to sample tissue along the parietal pleura and break apart a pleural adhesion through the use of the flexible bronchoscope (see Figures 5 and 6).
By using the redirecting stylet, the steerable chest tube could be repositioned into all dependent areas where fluid collected. Approximately 450 of the 500 ml of saline instilled prior to the procedure was removed through the steerable chest tube.
Discussion
This pilot animal study provides proof of principle for the use of the steerable chest tube to access and maneuver within the pleural space, drain pleural fluid, and take forceps biopsies. Aside from the steerable chest tube, no additional endoscopic equipment was necessary aside from what is used for standard bronchoscopy with forceps biopsies. This novel technique involved two commonly used skill sets (flexible bronchoscopy and tube thoracostomy) and incorporated a modified Seldinger technique to provide a minimally invasive means to access the pleural space and have the potential to perform diagnostic procedures.
The use of a steerable chest tube placed via Seldinger or surgical technique, a standard flexible bronchoscope, and a redirecting stylet may provide a minimally invasive alternative to adding additional procedures. A primary advantage of larger thoracoscopes is the ability to take larger biopsy samples. While larger samples were viewed as necessary in the past, it has been shown recently that a high diagnostic yield for malignancies and for mutational analysis can be achieved from smaller samples, even ones obtained through flexible bronchoscopy with fine needle aspiration (FNA).16,17 It is unknown what the effect of smaller pleural biopsies will have on diagnostic yield, but this should be a topic for future research.
A significant finding in the study was the ease at which the steerable chest tube could be redirected using the combination of curved metal redirecting stylet and an outer barrier sleeve around the tube. In this study, this feature overcame the previously noted lack of maneuverability when using a flexible bronchoscope in the pleural space.
Aside from the advantage of bronchoscopic access to the pleural space, the steerable chest tube has a distinct advantage over standard chest tubes. In cases of loculated fluid or air collections, the tube can be redirected so that an additional tube may not be needed and patients may avoid the added pain of multiple chest tubes.
As a future area of clinical research, this steerable chest tube may be evaluated as a means to enhance intrapleural treatments such as fibrinolytics, and may be compared with other techniques such as addition tube placement, pleuroscopy, or VATS. These uses were not the goals of this study, so, future studies will be needed to investigate the diagnostic yield of biopsy samples obtained by this technique. There are also therapeutic procedures such as talc insufflation for pleurodesis commonly performed through medical thoracoscopy, which were not the focus of this experiment and may be considered as a future use for the steerable chest tube.
There are limitations to this study. The diameter of the bronchoscope and its working channel limit the size of the forceps that can be used (though a standard bronchoscopic forceps can be used). In addition, while the redirecting stylet improves the maneuverability of the flexible scope, the flexible scope may not allow forceps to peel off pleural biopsies as easily as can be done with the rigid thoracoscope. Thus, it remains to be seen if pleural biopsies via this technique will be adequate for diagnosing malignancy and other pleural disorders to the degree that biopsies are from standard medical pleuroscopy. In addition, sterility must be maintained with the flexible bronchoscope, so steam sterilization (autoclave), with ethylene oxide (ETO) or plasma sterilization, will be required.
It should be noted that a flexi-rigid pleuroscope advanced through a trocar can be steered, visualize pleura, biopsy different areas, and drain fluid collections. Therefore, the purpose of this new device is not to replace pleuroscopy. The proposed system may work for simple adhesions, but for more complex adhesions, dissecting through a bronchoscope is unlikely to be successful. In these scenarios, VATS/decortication may even be necessary. A future niche for this steerable system may only be in cases where tube thoracostomy is needed and diagnostic procedures performed with the bronchoscope through the tube may be complementary.
This system was only tested by three pulmonologists, which is an insufficient number to extrapolate how the general population of pulmonologists will perform with the device. Many practicing pulmonologists may not perform tube thoracostomy, as a survey of pulmonary/critical care fellowship showed only 65 % of programs reach the recommended number of tube thoracostomies to achieve competency.18
In summary, this study provides proof of principle that a novel steerable chest tube can be directed successfully within the pleural space and can be used with a flexible bronchoscope to further access and visualize the pleural space, locate and biopsy implanted markers on the parietal pleural space, and adequately drain fluid from the pleural space. This procedure was accomplished with the steerable chest tube, a standard flexible bronchoscope, a redirecting stylet, and flexible biopsy forceps. Further studies will be needed to evaluate the usefulness of this procedure in the clinical setting.