Systemic sclerosis (SSc) is an autoimmune disorder characterized by inflammation and fibrosis of the skin and other organs. It has a range of clinical manifestations, and the typical internal organs involved include the lungs, kidneys, heart, and gastrointestinal tract. Though it is an uncommon condition, the mortality rate of this disease is higher than any other rheumatologic condition,1 and the majority of deaths occurring in SSc are related to pulmonary manifestations, including interstitial lung disease (ILD) and pulmonary arterial hypertension.2 SSc is subdivided into two subtypes, limited cutaneous SSc (lcSSc) and diffuse cutaneous SSc (dcSSc), based on the extent of skin involvement, with limited disease indicating that the dermatologic sclerosis is confined to the hands, distal extremities, face, and neck; while diffuse disease is characterized by more extensive sclerosis, especially of the internal organs such as the lungs.
With a prevalence of up to 90% of patients, ILD may complicate SSc as a result of lung inflammation and/or progressive fibrosis. ILD may occur with either SSc subtype, although more frequently with dSSc.3 Given the significant morbidity and mortality of SSc-associated ILD (SSc-ILD), recent studies have been performed in an attempt to identify novel treatment strategies to slow or reverse the progression of the disease. Nintedanib, a small molecule tyrosine kinase inhibitor, currently approved for the treatment of idiopathic pulmonary fibrosis (IPF) was recently shown to similarly reduce the rate of annual lung function decline in patients with SSc-ILD.4 Here, we discuss the pathogenesis of SSc-ILD and its common clinical manifestations. The objective of this article is to review the current therapies for SSc-ILD and discuss the role of nintedanib in the treatment of these patients.
Pathogenesis of systemic sclerosis-associated interstitial lung disease
The pathogenesis of SSc-ILD is a complex process that involves cellular and humoral immune dysregulation, inflammatory mediators, and fibrotic mechanisms.5 Patients who develop SSc could have underlying genetic predisposition to the disease with an environmental trigger that actually initiates the disease process.6 Similar to IPF, the initial injury begins in the alveolar epithelium and endothelium, followed by a cascade of both innate and adaptive immunological responses. Proinflammatory cytokines such interleukin-8 and tumor necrosis factor-α are released during the inflammatory phase. Activated macrophages, among other cells, also release profibrotic factors such as transforming growth factor-β, connective tissue growth factor, and platelet derived growth factor (PDGF).7 Differentiation and proliferation of myofibroblasts under the influence of these profibrotic factors, key effector cells well-known in the pathogenesis of IPF,8 have more recently been shown play a crucial role in the extracellular matrix (ECM) remodeling and disease progression in SSc-ILD.9 Fundamental to fibrotic ILDs, myofibroblast activation and excessive ECM deposition results in a perpetual self-sustaining cycle of fibrosis.
Vascular involvement is another key driver of SSc and likely contributes to the majority of organ involvement that occurs. Endothelin-1 has known vasoconstrictive properties but has also been shown to have mitogenic effects and enhancement of collagen synthesis.10 It is thought to be important in both the vascular and fibrotic changes that occur in SSc.11 Endothelin-1 has been noted to occur in higher concentrations in patients with SSc when compared with healthy control subjects and was inversely correlated with diffusion capacity.12 Endothelial cell apoptosis may contribute further to perpetuation of tissue injury/repair processes, but its role is incompletely understood. While this process has greater involvement in development of pulmonary hypertension, it is thought to be important in both the vascular and fibrotic changes that occur in SSc.11
Clinical considerations
ILD is the most common pulmonary manifestation of SSc. The reported prevalence of ILD in patients with SSc may be up to 90%,3 but varies depending on the distribution of serological pattern, definition of ILD, and the population studied (e.g. lcSSc, dcSSc, or a combination). While restrictive lung disease does occur in both dcSSc and lcSSc, it appears to occur with higher frequency in dcSSc.13 The incidence of ILD is highest among the subgroup of patients with anti-topoisomerase I antibody
(anti-Scl-70) positivity and dcSSc.14 Presence of anti-Scl-70 antibodies has been associated with lower and more rapid decline in forced vital capacity (FVC), while presence of anti-centromere antibodies has been associated with less restriction, isolated diffusion defects, and pulmonary hypertension.13,15 Radiographic findings of ILD have also been shown to be less frequent in the presence of anti-centromere antibodies.13 Additional patient characteristics such as gender and race may potentially contribute to the development of ILD among patients with SSc.16,17
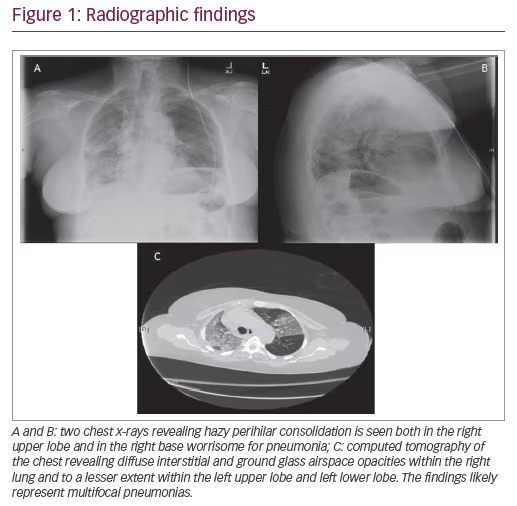
Presenting symptoms generally include dyspnea, fatigue, reduced exercise capacity, and cough. Exam findings, including fine, bibasilar, and inspiratory crackles, may suggest underlying ILD, and should prompt further evaluation with pulmonary function tests (PFTs), high-resolution computed tomography (HRCT) chest scans, and/or echocardiogram. PFT abnormalities including restriction and diffusion capacity deficits have been demonstrated in >70% of patients, although several patients may have normal lung function in the presence of early ILD. Radiographic findings consistent with fibrosis were seen in at least one-third of patients with scleroderma,18 while some other studies have reported prevalence up to 90%.3 Nevertheless, given the progressive nature of pulmonary fibrosis, early evaluation with HRCT is warranted, particularly among patients with risk factors. The most common pattern of fibrosis on HRCT in SSc-ILD is nonspecific interstitial pneumonia (NSIP) with ground-glass opacities and subpleural sparing.19 Usual interstitial pneumonia pattern, characterized by basal predominant peripheral reticulations, traction bronchiectasis, and honeycomb changes, is present in one-third of these patients and portends a poorer prognosis.20,21 The NSIP histology tends to be the fibrotic form more often than cellular NSIP, but the prognosis has not been shown to differ between the types of NSIP.21 Among patients with a histopathologic pattern of usual interstitial pneumonia, SSc is associated with increased presence of lymphocytic infiltrates compared to those with IPF.
Current therapies
Therapies for ILD related to SSc have traditionally focused on reducing the chronic inflammation that is believed to lead to fibrosis. These often include corticosteroids and other immunosuppressive agents. Despite these therapies, 3-year survival in patients with serious cardiac or pulmonary involvement is only about 50%.22 Physiologic parameters, including FVC, appear to be the best method of monitoring progression of fibrotic ILD, and most studies use FVC as the primary endpoint in evaluation of potential therapies for SSc-ILD.23 Key positive randomized control trials in SSc-ILD are outlined in Table 1.
Mycophenolate mofetil (MMF) is currently considered the first-line therapy for SSc-ILD based on the Scleroderma Lung Study (SLS)-II. This study was a multicenter, randomized, double-blind, parallel-group trial in 126 patients with SSc-ILD that compared the efficacy and safety of MMF (target dose of 1,500 mg twice daily) for 24 months versus oral cyclophosphamide (target dose 2 mg/kg per day) for 12 months followed by placebo for 12 months. Although there was no significant difference in the primary endpoint of change in FVC (percent predicted) at 2 years between groups, both groups demonstrating a significant increase in FVC, fewer patients stopped MMF prematurely and there were fewer treatment-related serious adverse events in patients on MMF compared to cyclophosphamide.24 Based on the SLS-I and II trials, cyclophosphamide is considered as an alternative therapeutic option.25,24 In SLS-I, cyclophosphamide showed modest improvements in lung volume, dyspnea score, quality of life, and HRCT score compared to placebo when studied over 12 months.25,26 However, treatment with cyclophosphamide may be complicated by significant serious adverse events, including hemorrhagic cystitis, neutropenia,
opportunistic infections, and bladder cancer; thus, making it a less appealing first-line agent.
There are very limited data to support the efficacy of corticosteroids in SSc-ILD,27,28 but rather evidence of risk of scleroderma renal crisis, particularly with higher doses.29,30 While a majority of patients receive corticosteroids at some point in their disease course, only a few small studies have shown marginal benefit of corticosteroid use over no immunosuppression.31,32 In a subgroup of patients with significant inflammation and ground-glass opacities on HRCT at presentation, corticosteroids might be utilized in conjunction with MMF titration. The generally accepted clinical approach focuses on cautious utilization of corticosteroids during acute exacerbations but is not recommended as a monotherapy in patients with SSc-ILD.
Azathioprine has been considered in combination therapy with cyclophosphamide,33 but the evidence to use it as monotherapy is less compelling. Finally, rituximab, a monoclonal antibody that targets CD20-positive B-lymphocytes, has been employed among patients with refractory or progressive ILD but the paucity of randomized controlled trials evaluating the efficacy of rituximab in SSc-ILD limits its utility. While these traditional immunosuppressive therapies have focused primarily on chronic inflammation in SSc-ILD, the advent of antifibrotic therapies approved for IPF have helped usher in a paradigm shift in the management of SSc-ILD.
Nintedanib and its mechanism of action
Nintedanib, an intracellular tyrosine kinase inhibitor, was shown to reduce the rate of FVC decline in patients with IPF in the INPULSIS trials (ClinicalTrials.gov identifier: NCT01619085)34 and was approved for treatment of IPF at a dose of 150 mg twice daily. Several in vitro studies show that nintedanib has antifibrotic, anti-inflammatory, and vascular remodeling effects with resultant attenuation of progression of fibrosis in preclinical models of IPF.35 Given the considerable similarities in the pathogenesis of IPF and SSc-ILD, particularly myofibroblast differentiation and dysregulated ECM deposition, it has been hypothesized that a similar effect of nintedanib may be seen in SSc-ILD and has been reported in in vitro studies of SSc-ILD and SSc skin fibrosis. Nintedanib was shown to inhibit the differentiation and migration of lung fibroblasts derived from patients with SSc-ILD; it also blocked PDGF-induced differentiation of normal lung fibroblasts to myofibroblasts, with resultant reduction in ECM expression.36
Nintedanib has also been shown to have similar effects on dermal fibroblasts obtained from patients with SSc compared to healthy subjects.37 In this study, nintedanib also prevented bleomycin-induced skin fibrosis and ameliorated fibrosis in a chronic graft-versus-host disease model and in tight-skin-1 mice. Reduction in pulmonary and dermal fibrosis demonstrated by decreased myofibroblast counts and ECM deposition has also been reported in nintedanib treated fibrosis fos-related antigen 2 (Fra2)-transgenic mice.38 Additionally, a significant effect on pulmonary vascular remodeling via inhibition of the proliferation of pulmonary vascular smooth muscle cells in these Fra2-transgenic mice, was demonstrated with nintedanib.38
Nintedanib—evidence from clinical trials
Based on the rationale that nintedanib can modulate the fundamental process of myofibroblast differentiation and ECM deposition in all ILDs, The Safety and Efficacy of Nintedanib in Systemic Sclerosis (SENSCIS) trial (ClinicalTrials.gov identifier: NCT02597933) recently evaluated use of nintedanib in patients with SSc-ILD. This was a multinational, randomized, double-blind, placebo-controlled, parallel-group trial that included 576 adult patients diagnosed with SSc with HRCT chest scan showing fibrosis affecting at least 10% of the lungs, FVC of at least 40% predicted value, and the diffusing capacity of the lungs for carbon monoxide (DLCO) of 30–89% predicted value. Four of the 819 patients screened had clinically significant pulmonary hypertension defined by either clinical or echocardiographic evidence of right heart failure, cardiac index ≤2 L/minute per square of body surface area, or history of parenteral therapy with vasodilators, and were excluded from the study. Patients were randomized to either nintedanib 150 mg twice daily or placebo with primary efficacy evaluation conducted at week 52. The primary endpoint was the annual rate of decline in FVC.
The trial investigators found the annual rate of change in FVC over 52 weeks to be lower in the nintedanib group (-52.4 ± 13.8 mL) compared with the placebo group (-93.3 ± 13.5 mL) with benefit becoming evident by week 12. Importantly, the benefit of nintedanib over placebo was present but less pronounced in patients taking mycophenolate at baseline compared with those not on mycophenolate. There was no improvement in health-related quality of life nor evidence that nintedanib modifies the course of other organs affected by SSc. Sixteen percent of patients in the nintedanib group experienced an adverse event that led to discontinuation compared with 8.7% of patients in the placebo group. Diarrhea was the most common adverse event, occurring in almost 76% of patients taking the drug, and was mild to moderate in most patients. About 5% of patients receiving nintedanib had elevations in aminotransferases reaching three times the upper limit of normal.4 On September 6, 2019, nintedanib was approved by the US Food and Drug Administration (FDA) as the first and only treatment for SSc-ILD.39
In addition, nintedanib was evaluated in patients with progressive fibrosing ILD, including SSc-ILD, in the INBUILD trial.40 This was a randomized, double-blind, placebo-controlled trial that included a total of 663 patients, of which 39 (5.9%) had SSc-ILD. Key inclusion criteria were fibrosing lung disease with >10% of lung volume affected based on HRCT, confirmed disease progression despite use of conventional therapies within 24 months prior to enrollment, FVC of at least 45% predicted, and DLCO of 30–79% predicted. Patients were randomized to receive either nintedanib 150 mg twice daily or placebo. The primary endpoint was annual rate of FVC decline followed over 52 weeks. The results demonstrated a statistically significant reduction in the FVC rate of decline: -80.8 mL per year in the nintedanib group and -187.8 ml per year with placebo. The difference was more pronounced in patients with a usual interstitial pneumonia-like pattern of fibrosis. Diarrhea was the most common adverse event in the nintedanib group, and more people required dose reductions or discontinuation of nintedanib when compared with placebo.40
Treatment decision-making
The decision to initiate treatment in patients with SSc-ILD can be a challenging one that requires integration of several patient-related and disease-specific features. Although patients with SSc-ILD may have increased mortality rates, the currently available treatments have potentially significant toxicity with only modest efficacy. Survival prediction tools, such as those by Goh et al.,41 and the SADL model by Morisset et al.,42 may be helpful in predicting short-term mortality and aid in treatment decisions. With the advent of antifibrotic therapy, a nuanced understanding of the risks and benefits of all therapeutic options in a disease previously treated with only immunosuppression is crucial. There is increasing evidence emphasizing the importance of early treatment for ILD; larger centers are now focusing on annual screening of patients with SSc with PFTs and/or HRCTs. However, guidelines for recommendation of treatment algorithms are yet to be published.
Given the potential benefit from initial immunosuppressive therapy during the inflammatory phase in SSc-ILD, MMF remains the first line of therapy given its efficacy in modulating both pulmonary and extra-pulmonary manifestations of SSc.43,24 MMF therapy has been shown to improve lung function as measured by FVC while nintedanib only slowed down disease progression in the SENSCIS trial. Further, there was only a modest additional benefit of nintedanib to MMF therapy among patients with stable disease; the annual rate of change in FVC among patients on background MMF was -40.2 mL per year in the nintedanib arm compared to -66.5 mL per year in the placebo arm.4 In the absence of randomized controlled trials evaluating MMF or cyclophosphamide versus nintedanib, we believe that nintedanib should only be utilized among patients with SSc-ILD who decline, or are intolerant to, MMF and/or cyclophosphamide therapy or demonstrate disease progression despite these initial therapies. The benefit of treatment with nintedanib over placebo (± background MMF) was noticeable only beyond the 12-week mark4 and should be a consideration in the treatment algorithm. As mentioned earlier, patients with clinically significant pulmonary hypertension were excluded from the SENSCIS trial; therefore, the potential benefits of nintedanib in these patients is unknown. The majority of these patients require long-term maintenance therapy to preserve their lung function and should be monitored with PFTs every 3–6 months. Overall, nintedanib was tolerated at least as well as in IPF despite known gastrointestinal issues that are common among patients with SSc. The extension trial, SENSCIS-ON, will assess the long-term safety of nintedanib in SSc-ILD and may shed more light on its utility in clinical practice.
Conclusions
SSc-ILD is a chronic, progressive fibrotic lung disease associated with increased mortality. Clinical practice guidelines to best inform a therapeutic approach for these patients is absent; MMF is currently considered the standard of care based on the SLS-II. However, recent studies evaluating nintedanib in SSc-ILD have been encouraging and led to FDA approval of nintedanib as the first and only for SSc-ILD. Many key questions, such as when to initiate antifibrotic therapy, how best to measure the treatment response, and how long treatment should be continued, remain unanswered.