Chronic obstructive pulmonary disease (COPD) imposes a substantial health and economic burden worldwide and is a major cause of death.1 Clinical features of advanced COPD include severe airway obstruction and lung hyperinflation that can lead to chronic respiratory failure, which is associated with a poor outcome.2,3
Observational data has shown that non-invasive ventilation (NIV) is associated with improved quality of life (QoL) in patients with advanced COPD.4,5 The use of NIV has increased markedly in the last decade and is now considered standard of care in the management of acute chronic respiratory failure. However, home NIV for the treatment of chronic respiratory failure has had more widespread adoption in Europe than in the US. Clinical trials have failed to demonstrate a survival benefit when NIV was added to home oxygen treatment (HOT).6,7 In 2009, a study found a small survival benefit, but at the cost of worsening QoL.8
Concern was expressed that early studies had employed low pressure NIV and therefore did not treat chronic respiratory failure and improve hypercapnia. A retrospective study found that pressure-controlled NIV employing higher pressures with a high back-up rate was well tolerated and could improve the partial pressure of carbon dioxide (PaCO2) and lung function.9 In 2014, a multicentre, randomised, controlled trial was the first to conclusively demonstrate that the addition of long-term NIV to standard treatment improves survival of patients with hypercapnic stable COPD. This survival benefit became evident over 1 year of treatment. Furthermore, the addition of NIV to standard treatment resulted in a significant improvement in health-related QoL.10 However, this study did not demonstrate any effect of NIV on hospitalisation. A 2013 Cochrane review concluded that future studies should focus on adequate patient selection, ventilator settings, training and length of ventilation, as well as exacerbation frequency, admissions to hospital and survival.11
Choosing the optimal strategy for NIV in patients with acute exacerbations of COPD is particularly problematic. These episodes are characterised by deterioration in gas exchange accompanied by a worsening in the clinical condition of the patient, leading to hypercapnia, acidosis and hypoxemia with resulting clinical deterioration in cardiovascular and neurological function.12 Hospital readmissions and mortality in these patients is high,13 leading to a number of studies investigating the use of NIV in acute respiratory failure. A 1995 study established NIV as the gold standard to treat acute hypercapnic respiratory failure. The use of NIV reduced the need for endotracheal intubation, length of the hospital stay and in-hospital mortality rate.14 The latest clinical study investigating NIV in acute exacerbations of COPD was the HOT-home mechanical ventilation (HMV) phase III clinical study. A symposium presented at the European Respiratory Society (ERS) Annual Congress held in London, UK , 3–7 September 2016, discussed the findings and implications of the HOT-HMV study. This article presents the proceedings of the symposium.
Home Oxygen and Home Mechanical Ventilation Following Life-threatening Exacerbations of COPD – Results from HOT-HMV UK
Presented by: Patrick Murphy
Consultant in Respiratory Medicine, Lane Fox Clinical Respiratory Physiology Research Centre, Guy’s & St Thomas’ NHS Foundation Trust,
London, UK
Despite advances in the use of NIV, outcomes remain poor following acute hypercapnic exacerbations of COPD, with 40% mortality at 3 months.15 Until recently, there have been few effective treatment options for these patients and clinical data have failed to demonstrate an additional benefit to standard care for NIV. The 2014 RESCUE study found that the addition of NIV for 1 year to standard treatment of COPD patients with prolonged hypercapnia did not show any improvement in terms of time to readmission or death, but did improve daytime PaCO2, night-time transcutaneous CO2 and health-related QoL, suggesting that the intervention warranted further investigation.16 It was considered that data in support of the use of NIV could be improved with appropriate patient selection and use of therapy.
The HOT-HMV trial was based on the hypothesis that HMV titrated to abolish nocturnal hypoventilation would improve admission-free survival (increase time to readmission or death) following an acute life-threatening exacerbation of COPD in patients with persistent hypercapnia.17 The study was a multicentre, open-label, randomised, controlled trial that was powered using real-world data from the Leeds NIV cohort (unpublished data), which had a readmission rate of 55% at 1 year. It was assumed that the use of NIV would be able to reduce this rate to 25%. It was also assumed that the dropout rate would be around 22%, an assumption that is supported by previous studies,16 yielding a statistical power of 80% and significance of 0.05 with a study population of 116 patients randomised 1:1 via minimisation. To prevent imbalances that might affect the primary outcome, minimisation was performed for age (<65 versus ≥65 years), body-mass index (BMI, ≤20 versus >20 kg/ m2), current long-term oxygen therapy (LTOT) use prior to randomisation (yes versus no), frequency of COPD-related admissions in previous 12 months (<3 versus ≥3) and centre of recruitment.
The index admission was an acute exacerbation of COPD requiring NIV. When the patients’ pH had improved to greater than 7.3, there was a recovery period of 2 weeks to determine whether patients had ongoing persistent hypercapnia. If, after this time, patients had a PaCO2 exceeding 7 kPa, they were randomised to HOT only or HMV in addition to HOT (Figure 1). HOT was administered at the lowest level that could improve the partial pressure of oxygen (PaO2) to greater than 8 kPa and then HMV was titrated to abolish nocturnal hypoventilation. The inclusion criteria were: patient admission with an acute hypercapnic exacerbation of COPD; a smoking pack-year history ≥20; forced expiratory volume (FEV1) <50%; FEV1/forced vital capacity (FVC) <60%; persistent hypercapnia at 2 weeks following resolution of hypercapnic acidosis (pH ≥7.3; PaCO2 ≥7kPa; PaO2 <7.3 kPa or >7.3 and <8.0 kPa with

secondary polycythaemia; pulmonary hypertension; peripheral oedema or significant nocturnal hypoxia, oxygen saturation [SaO2] <90% for >30% sleep time). Exclusion criteria were: patients unable to wean off NIV prior to discharge/transfer due to persistent hypercapnic respiratory failure with pH <7.3 despite adequate NIV; patients requiring daytime NIV or >6 hours of nocturnal NIV to maintain a pH ≥7.3; development of worsening hypercapnic respiratory failure with acidosis during initiation of oxygen therapy (arterial blood gases [ABG] – pH <7.3 taken 2–4 hours after waking); primary diagnosis of restrictive lung disease causing hypercapnia; significant symptomatic obstructive sleep apnoea contributing to patient morbidity; significant obesity (BMI >35 kg/m2); assessment more than 4 weeks from resolution of index exacerbation; inability to consent or comply with the trial protocol; post-extubation or decanulation; inability to tolerate NIV (if given) during acute illness; other morbidities that might affect the outcome such as unstable coronary artery syndrome and renal replacement therapy; age <18 years and pregnancy. In essence, the target population were patients with severe COPD and a recent life-threatening exacerbation, with chronic hypercapnic respiratory failure (PaCO2 >7 kPa) in the recovery phase and without other significant cause of sleep disordered breathing respiratory failure. The intervention was administered in the recovery phase before established restabilisation. The cut-off of pH 7.3 was chosen rather than the conventional cut-off of pH 7.35 because it was considered that at that point, patients were no longer in the acute phase and were clinically stable.
The primary outcome measure was 12-month admission-free survival, i.e., time to readmission or death within 12 months. Other secondary outcomes included health status and readmissions, other forms of exacerbations, ABG, sleep (actigraphy and overnight oximetrycapnography) and QoL measures such as the severe respiratory insufficiency (SRI) questionnaire, St George’s respiratory questionnaire (SGRQ) and the EuroQoL five dimensions questionnaire (EQ5-D). Oxygen was initiated in the daytime at the minimum rate required to correct hypoxia, with the aim of achieving PaO2 in excess of 8 kPa. A high-pressure, high-intensity strategy as previously advocated by German research groups10,18,19 was adopted in a modified form, targeting amelioration of nocturnal hypoventilation, but without a high backup rate as this was not considered necessary. Initial daytime acclimatisation to NIV occurred with overnight titration of settings starting with an inspiratory pressure of 18 cmH2O and aiming for a treatment pressure of 25 cmH2O if needed to correct hypoventilation (measured by transcutaneous CO2).
Recruitment took place between 2010 and 2015, and involved the screening of 2,021 patients. Of these, a total of 1,905 were excluded from the study. Around a third declined to participate (n=296,16%), were unable to give consent (n=237, 12%) or died prior to screening (n=128, 7%). Among the others, around half of the remaining patients (n=419, 22%) had significant improvements in their daytime arterial gases, therefore did not have persistent hypercapnia and did not meet the inclusion criteria. In addition, 252 (13%) were unable to wean from NIV; 157 (8%) were admitted for reasons other than an acute exacerbation of COPD; 131 (7%) were unable to tolerate NIV; 76 (4%) had obstructive sleep apnoea; 95 (5%) had a BMI exceeding 35 kg/m2; 51 (3%) had post decanulation or extubation on index admission; 46 (2%) were unable to screen within the trial protocol and 8 (<1%) were decompensated with oxygen therapy, with other reasons cited in the remaining 8 (<1%). It is worth highlighting that around 50% of the patients suitable for assessment did not have persisting hypercapnia and only around 15% of the screened patients were recruited, as this represents a substantial contrast with previous studies.16 Another noteworthy finding was that the absolute dropout rate was 16%, well within the 22% assumption in the power calculation. Furthermore, before the primary outcome the dropout rate was only 10% and no patients were lost to follow-up.
Baseline characteristics were generally typical of those seen in clinical practice: median age 66.7 years, median BMI 21.1 kg/m2 (lower than average because of the exclusion of very obese individuals), half female and half male, with significant airflow obstruction (FEV1 around 0.6 L), and no significant sleep apnoea. ABG analysis on room air following a night of oxygen therapy without NIV showed PaO2 of 6.4 kPa and PaCO2 of 7.9 kPa. The two patient groups were well matched, with no significant difference in baseline characteristics. They also had significant impairments in health-related QoL, assessed by SGRQ, SRI questionnaire and Medical Research Council (MRC) dyspnoea score. Baseline sleep studies revealed no significant obstructive sleep apnoea in these patients.
The intervention in the HOT-HMV group was a median 1 L/min (0.5 to 1.5 L/min) of oxygen with a median inspiratory positive airway pressure (IPAP) of 24 cmH2O and expiratory positive airway pressure (EPAP) 4 cmH2O. The median backup rate was 14.0 bpm.
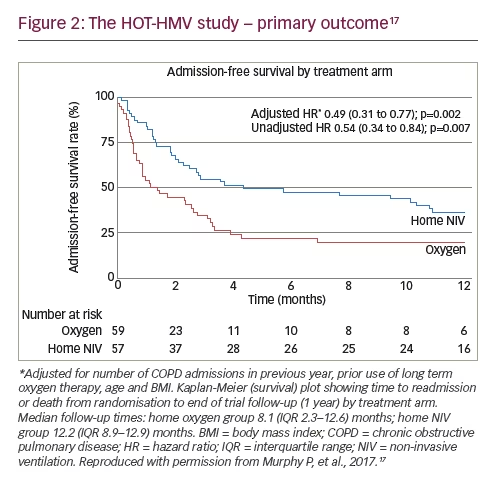
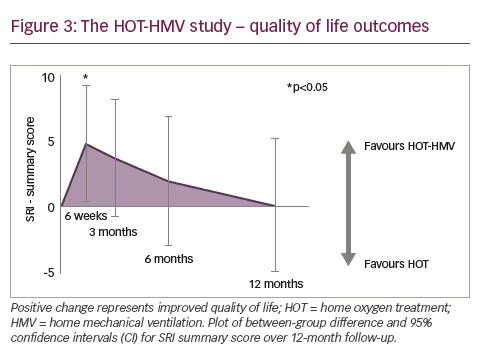
The total number of patients meeting the primary endpoint showed significant difference at 12 months: 38 (67%) in the HOT-HMV group versus 42 (71%) in the HOT group. Patients receiving HMV as well as HOT had a median admission-free survival of 4.3 months compared with 1.4 months in those receiving HOT alone (Figure 2). This gave an adjusted hazard ratio (HR) of 0.49 (p=0.002). The data were also analysed in terms of number needed to treat, and it was established that six patients need to be treated with HMV and HOT to prevent one readmission or death over 12 months, a significant positive finding. It was important to demonstrate that the patients were ventilated. Analysis of mean transcutaneous CO2 and peak transcutaneous CO2 showed a significant treatment effect of around 1 kPa (p<0.001). This translated to improvements in ABG: at 3 months, the adjusted effect showed a reduction of PaCO2 of 0.53 kPa (p=0.015). This was not statistically significant from 6 months onwards because of the diminution in numbers; however, a treatment effect could still be detected at 12 months. Another important finding was the impact of the intervention on health-related QoL since previous studies had reported a reduction in QoL in the NIV group.8 Using the SRI score, significant benefits were reported within the first 6 weeks. These benefits reduced over time and there was no statistically significant difference between intervention and control groups at prolonged follow-up but there was no suggestion of worsened QoL in the intervention group (Figure 3).
Further examination of the primary endpoint of admission-free survival yielded interesting findings. The number of deaths (28% in the HOT-HMV group versus 32% in the HOT group) did not differ significantly between the two groups. The causes of death were primarily respiratory (COPD in 21 patients, pneumonia in six, respiratory failure in four, lung cancer in two, cor pulmonale in one and congestive heart failure in one). A comparison of exacerbations was performed with a reduction in the rate of all exacerbations demonstrated, not only those requiring hospitalization. The median exacerbation rate per year was 3.84 (1.68–6.02) in the HOT-HMV group versus 5.06 (0.99–9.19) in the HOT only group (adjusted HR 0.66, p=0.026).
In conclusion, outcomes remain poor following an acute life-threatening exacerbation but are significantly improved by the addition of HMV to HOT. This trial has shown that persistent hypercapnia is an important clinical feature and systematic screening of patients is required following acute NIV to identify patients with COPD most likely to benefit from HOTHMV in terms of time to readmission or death within 1 year.
Lessons Learned from the HOT-HMV Trial – What are the Clinical Implications?
Presented by: Nicholas Hart
Professor of Respiratory and Critical Care Medicine, Lane Fox Clinical Respiratory Physiology Research Centre, Guy’s & St Thomas’ NHS Foundation Trust, London, UK
Professor Hart’s presentation considered the background of the HOT-HMV trial and the factors in the trial design that aimed to maximise its impact and led to the successful outcome, in particular the low dropout rate. The HOT-HMV trial17 was initiated following the introduction of the 2007 UK Global Medical Excellence Cluster initiative,20 with respiratory medicine being identified as one of the clusters. This led to an increase in research in COPD.
One important factor in the trial design regards the target population. The study participants were very sick patients with COPD with very limited treatment options other than oxygen therapy.17 All were on nebulisers, long-acting beta agonists, anticholinergics and inhaled corticosteroids. There was concern that these patients may have been too sick to respond to treatment. Other key considerations in the trial design included the intervention. In this study, oxygen therapy was defined as HOT as opposed to LTOT. The terminology ‘home oxygen therapy’ seemed more appropriate in a group of patients that lacked clinical stability. In terms of NIV, other studies have employed high-intensity and high-pressure NIV with a high backup rate. To determine the optimum strategy for this study, a randomised crossover trial was undertaken by the HOT-HMV trial research team. This study found that a high-pressure ventilation strategy was not better or worse than a high-intensity strategy.21 Furthermore, a ventilator with a high backup would be very expensive. Since this intervention could be potentially used in a high patient population, an inexpensive ventilator was essential; hence a high-pressure technique was chosen. The successful Kohnlein study used moderate pressure.10 The parameters chosen in this study were between the Kohnlein approach and the high-intensity approaches.
The use of a sham device was also considered, but clinical concern was raised that an increase in dynamic deadspace with zero pressure support would have a detrimental effect on unstable patients with COPD.22 In addition, blinding was considered; however, the patient knows they are on ventilation so blinding was not considered feasible.23 A crossover into the treatment arm was also an area of concern. The purist approach of maintaining these patients on HOT alone was balanced by concerns, based on clinical experience, that patients might require multiple readmissions with acute exacerbations of chronic respiratory failure. As the patients would then have reached the primary endpoint and the trial was to be analysed on an intentionto- treat basis, it was agreed that HMV could be added to HOT if both the clinician and patient considered this appropriate, although this could potentially dilute the treatment effect. A total of 17 patients underwent such a crossover.
The primary outcome of this study was very strong as readmission into hospital was important to the patient.17 The key messages of the study were that HOT-HMV treatment reduced the likelihood of readmission or death by almost 50%, and that HOT-HMV increased the time to readmission or death by over 90 days. A post hoc analysis found that 28-day readmission was also reduced by two-thirds in patients receiving HMV and HOT compared with HOT alone, an important consideration for healthcare systems which impose penalties for readmission within this time frame.
It is useful to compare the HOT-HMV trial with previous studies, in particular the RESCUE trial,16 to enable us to improve the design of future trials. The RESCUE trial enrolled patients with a less stringent PaCO2 criterion (daytime PaCO2 >6 kPa) following cessation of acute NIV during the acute inpatient admission. By contrast, the HOT-HMV trial enrolled patients only if the daytime PaCO2 exceeded 7 kPa at least 2 weeks post resolution of acidosis. The HOT-HMV trial enrolled patients with persistent hypercapnia and therefore chronic respiratory failure, which is a group who have previously been shown to benefit in physiological studies.24,25 According to its inclusion criteria, the RESCUE trial is likely to have allowed patients with potentially reversible hypercapnia and therefore a better prognosis.26 The RESCUE trial also showed a reduction in daytime PaCO2 in the control group during the follow-up period, confirming resolution of hypercapnia during an expended recovery phase. By contrast, the HOTHMV trial screening data showed that more than half of the screened patients were ineligible for randomisation due to improved PaCO2 by the time of trial assessment. These screening data have important clinical implications. It is hoped in the future to develop predictive modelling to understand which patients physicians should be treating at 2–4 weeks, potentially facilitating earlier intervention with NIV.
In terms of other clinical implications, patients with COPD with chronic respiratory failure and persistent hypercapnia have a poor outcome. Clinicians would need to add HMV to HOT in only six patients to prevent one admission to hospital or one death within the following year. It has been shown that the major driver of the improvement in the primary outcome measure was admission avoidance, which has major clinical relevance, as admission avoidance is beneficial to the patient and the healthcare system. Patients with COPD deteriorate every time they have an exacerbation that results in hospital readmission.27 Previous data showed that median time to admission was 32 days.24 A post hoc analysis demonstrated that patients receiving HMV and HOT were two-thirds less likely to be re-admitted within 28 days compared with those receiving HOT alone.
The results of the study will drive change in the clinical management of patients with severe COPD and chronic respiratory failure following a life-threatening exacerbation. Indeed, a post-acute NIV clinic, which runs twice a month, screens patients with COPD for persistent hypercapnia 2–4 weeks following discharge from hospital. If the PaCO2 exceeds 7.0 kPa and the PaO2 is less than 7.3 kPa, this should prompt the clinician to consider initiating HMV in addition to HOT. The data support the initiation and inpatient titration of high-pressure NIV in patients with COPD who remain persistently hypercapnic 2–4 weeks after cessation of acute NIV.
Home NIV for COPD – Where are we now?
Presented by: Michael Dreher
Professor of Medicine/Pneumology, Division of Pneumology, University Hospital Aachen, Germany
Professor Dreher began by assessing the evidence for NIV in the treatment of chronic hypercapnic respiratory failure due to COPD. The Kohnlein study was targeted to reduce baseline PaCO2 by at least 20% or to achieve PaCO2 values lower than 6.5 kPa. This was achieved with a mean IPAP of 21.6 ± 4.7 mbar, EPAP of 4.8 ± 1.6 mbar and a breathing frequency of 16 bpm. These interventions resulted in a change in PaCO2 over time, with a greater reduction in patients receiving NIV (termed NPPV [non-invasive positive pressure ventilation] in this study).10 Previous studies also showed that it is possible to reduce CO2 by NPPV: high-pressure and low-pressure ventilation were compared in a randomised crossover trial resulting in a significant reduction of PaCO2 with high-pressure NPPV.18 When patients crossed over to lowpressure ventilation, their PaCO2 increased. Furthermore, compliance was 3.6 h/night longer in patients receiving high-pressure ventilation compared with those receiving low-pressure ventilation. Only highpressure NPPV resulted in significant improvements in exercise-related dyspnoea, QoL and various lung function parameters.18
The Kohnlein study provided clear evidence that NPPV improved QoL; a difference of 5.6 points (95% confidence interval [CI] 0.1–11.1) was reported in favour of the NPPV group (p=0.0445).10 This finding is supported by an earlier German multicentre study, which showed that general and condition-specific health-related QoL aspects significantly improved following 1 month of HMV, and remained stable over 12 months.4
The main outcome measure in the Kohnlein study was mortality rate. A significantly lower mortality rate was seen in the NPPV group compared with the control group (Figure 4).10 In 2015, an expert panel recommended that NIV should be considered in patients with COPD with a waking PaCO2 >6.7 to 6.9 kPa, an overnight PaCO2 >7.3 kPa, or both, who are symptomatic and compliant with other therapies.28

Exercise capacity is also an important consideration in assessing interventions for COPD. Three studies have shown that exercise capacity can be improved by NIV.29–31 A 2014 meta-analysis evaluated exercise training with NIV in terms of physiologic effects after the completion of a pulmonary rehabilitation programme.32 The analysis did not show improved respiratory outcomes with NIV but the authors concluded that further investigation was warranted, given the small number of available studies, small sample size and absence of power calculation of available studies.
NIV also plays an important role in pulmonary rehabilitation. In a 2008 study, patients with chronic respiratory failure due to COPD (n=72) were randomised to nocturnal NPPV in addition to rehabilitation or rehabilitation alone. Addition of NPPV (IPAP of 20 mbar, breathing frequency 18 bpm) was associated with improvements in QoL parameters and a significant reduction in PaCO2 levels.33
In the future, many areas warrant further investigation. A recent study investigated the impact of NIV (23 mbar) on the levels of cytokines and established cardiovascular biomarkers. Significant reductions in levels of pro-brain natriuretic peptide were seen (mean reduction of 578 ± 1332 ng/L) after 3 months of NPPV (p=0.017 versus baseline).17 In 2015, another study (n=64) investigated the hypothesis that daily variations in three parameters recorded by NIV software: respiratory rate (RR), percentage of respiratory cycles triggered by the patient (%Trigg) and NIV daily use, might predict exacerbation risk. The study detected and medically confirmed 21 exacerbations and concluded that daily variations in RR and %Trigg are predictors of an exacerbation.34 These findings have important implications: it may be possible in future to predict exacerbations using telemonitoring, initiate early treatment and prevent rehospitalisations for exacerbations. However, in 2016, a European Respiratory Society Task Force published a consensus statement concluding that at present, there is insufficient evidence to recommend telemonitoring and that more research is needed.35
In conclusion, there is good evidence to support the use of home NIV for patients with COPD and chronic hypercapnic respiratory failure. In terms of facilitating exercise capacity, NIV is an option for some patients experienced in its use. It is not generally indicated due to the lack of conclusive evidence. Good evidence is available to support the use of home NIV to promote pulmonary rehabilitation in patients with chronic hypercapnic COPD. In addition, home NIV is indicated after acute respiratory failure in patients with prolonged hypercapnic respiratory failure. In the future, there is a need to phenotype patients with COPD who will benefit most from long-term NIV. There is also a need to investigate further the use of telemonitoring in detecting exacerbations.
Discussion and Conclusion
It is generally agreed that the debate on high-intensity NIV has been resolved and that high-pressure NIV is the optimum strategy for the treatment of chronic respiratory failure in patients with COPD. The goal of NIV should be to reduce PaCO2. This can be achieved using a range of IPAP levels and it is important to adequately ventilate the patient. In future, physiologically targeted lower pressure ventilation may be possible with advances in ventilator technology. Indeed, if we set EPAP and abolish expiratory flow limitation, the driving pressure could be reduced and adherence would be expected to be enhanced. In the HOT-HMV trial, patients increased their use of HMV from 4 to 7 hours and tolerated the treatment more as the study went on.17
In conclusion, the addition of home NIV to HOT in patients with COPD and persistent hypercapnia following a life-threatening exacerbation increased the time to readmission or death. The HOT-HMV trial has the potential to change clinical practice and these findings are expected to have substantial implications as well as inform the design of future studies.