Asthma is a heterogeneous disease with notable variation in its clinical course and response to treatment. Despite management with standard-of-care therapies, a proportion of patients remain uncontrolled and at risk of life-threatening exacerbations and disease worsening. Advances in understanding the heterogeneity of asthma have helped to identify patients who will benefit from an understanding of the response to medications in individual patients and potential predictors of treatment response. This will theoretically lead to a more personalized approach to asthma care.
Over the past 20 years, attention has been directed to the introduction of a new class of medications, referred to as asthma biologics. Currently, there are five medications within this class approved for the treatment of asthma, including therapies individually directed at immunoglobulin E (IgE), eosinophils and certain interleukins, specifically the interleukin-4 (IL-4) and interleukin-13 (IL-13) pathways. Lebrikizumab is an antibody that binds to IL-13.1,2 It has undergone several trials in asthma with mixed results and has also been studied for the management of atopic dermatitis.1,2
Q. What is the mechanism of action of lebrikizumab?
IL-13 is a pleiotropic effector cytokine central to type 2 inflammation in severe asthma. It contributes to many of the characteristic features of asthma, including mucus production, eosinophilic airway inflammation, IgE synthesis, bronchial fibrosis and airway hyper-responsiveness. IL-13 is, therefore, recognized as a potential therapeutic target in patients with uncontrolled asthma and higher concentrations of type 2 biomarkers.
Lebrikizumab is a humanized monoclonal antibody that binds to soluble IL-13 with high affinity and blocks signalling through the active IL-4Rα/IL-13Rα1 heterodimer.1,2
Q. What were the aims, design and inclusion criteria of the LAVOLTA I and II trials, and what were the initial findings in terms of exacerbation rate reduction?
LAVOLTA I and II were replicate randomized, controlled, phase III trials designed to further assess the efficacy and safety of lebrikizumab in patients with uncontrolled asthma despite treatment with standard-of-care medications (ClinicalTrials.gov identifiers: NCT01867125; NCT01868061).3,4 The trials also assessed serum periostin concentration and blood eosinophil count as biomarkers for identifying patients who are most likely to benefit from lebrikizumab treatment.5 Blood eosinophils and serum periostin are two biomarkers associated with type 2 inflammation.
The two trials were multicentre, multinational, double-blind, placebo-controlled trials. Both studies consisted of at least a 2-week screening period, a 52-week placebo-controlled period, a 52-week active-treatment extension and a 20-week safety follow-up period. The findings of the 52-week placebo-controlled period were published in Lancet Respiratory Medicine in 2016 by Hanania et al.2
Adult patients with uncontrolled asthma, predicted pre-bronchodilator forced expiratory volume in 1 second (FEV1) of 40–80%, and stable background therapy were randomized 1:1:1 with an interactive voice-web-based response system to receive 37.5 mg or 125 mg lebrikizumab or placebo subcutaneously once every 4 weeks. Randomization was stratified by screening serum periostin concentration, history of asthma exacerbations within the last 12 months, baseline asthma medications and country. The primary efficacy endpoint was the rate of asthma exacerbations over 52 weeks in biomarker-high patients (periostin ≥50 ng/mL or blood eosinophils ≥300 cells per μL), analysed with a Poisson regression model corrected for overdispersion with Pearson’s Chi-square (χ2). That included terms for the treatment group, number of asthma exacerbations within the 12 months before study entry, baseline asthma medications, geographic region, screening periostin concentration and blood eosinophil counts as covariates.
Oone thousand and eighty-one patients were treated in LAVOLTA I, and 1,067 patients were treated in LAVOLTA II. Over 52 weeks, lebrikizumab reduced exacerbation rates in biomarker-high patients in the 37.5 mg dose group (rate ratio [RR] 0.49, 95% confidence interval [CI] 0.34–0.69; p<0.0001) and in the 125 mg dose group (RR 0.70, 95% CI 0.51–0.95; p=0.0232) versus placebo in LAVOLTA I. Exacerbation rates were also reduced in biomarker-high patients in both dose groups versus placebo in LAVOLTA II (37.5 mg: RR 0.74, 95% CI 0.54–1.01; p=0.0609; 125 mg: RR 0.74, 95% CI 0.54–1.02; p=0.0626).
Pooling both studies, the proportion of patients who experienced treatment-emergent adverse events, serious adverse events and adverse events leading to study drug discontinuation were similar between lebrikizumab and placebo. For both lebrikizumab doses, the following findings were reported:
-
79% of patients experienced treatment-emergent adverse events versus 80% for placebo;
-
8% of patients experienced serious adverse events versus 9% for placebo; and
-
3% of patients experienced adverse events leading to study drug discontinuation versus 4% for placebo.
The following serious adverse events were reported in the placebo-controlled period: one event of aplastic anaemia and five serious adverse events related to raised concentrations of eosinophils in patients treated with lebrikizumab, and one event of eosinophilic pneumonia in the placebo group.
Based on these results, the investigators concluded that lebrikizumab did not consistently show a significant reduction in asthma exacerbations in biomarker-high patients. However, lebrikizumab blocked IL-13 as evidenced by the effect on IL-13-related pharmacodynamic biomarkers, and clinically relevant changes could not be ruled out.
As a result of the inconsistent results, Roche (Basel, Switzerland) decided not to pursue medication labelling for asthma due to a financial decision based on a competitive market. Eli Lilly and Company, Inc. (Indianapolis, IN, USA) took over further evaluation of the compound for the treatment of atopic dermatitis and consideration for future studies in asthma. A recent study published in the New England Journal of Medicine reported on the efficacy of lebrikizumab in atopic dermatitis.6
Q. What was the rationale for investigating the effect of lebrikizumab on inflammatory biomarkers in patients with asthma, and what methodology was used in this analysis?
Further evaluation of the LAVOLTA I and II studies was conducted to determine the effect of lebrikizumab on selected biomarkers related to allergic inflammation. To date, there has been no published data on the impact of lebrikizumab on type 2 inflammatory markers. The results of this analysis were presented at the 2023 Annual Meeting of the American Academy of Allergy, Asthma and Immunology in San Antonio, TX, USA, on 26 February 2023 by Stanley J Szefler on behalf of his colleagues.* The abstract presentation was titled “Lebrikizumab Decreases Inflammatory Biomarkers in Patients with Asthma: Data from Randomized Phase 3 Trials (LAVOLTA I and II)”.5 This study was funded by Roche, and Eli Lilly and Company provided additional funding for statistical analyses.
The objective of this analysis was, therefore, to assess the effects of treatment with lebrikizumab on type 2 circulating inflammatory biomarker levels in patients with asthma. Four type 2 inflammatory biomarkers were chosen for analysis:
-
C-C motif chemokine ligand 13 (CCL13), a driver of chemotaxis in immune cells
-
C-C motif chemokine ligand 17 (CCL17), a driver of chemotaxis in T helper 2 cells
-
periostin, an IL-13 activator
-
IgE, a well-known hallmark of allergic inflammation.
Inflammatory markers were measured before randomization and at 1, 4, 24 and 52 weeks after randomization.
Q. What were the findings from your analysis?
Baseline characteristics of the patients in the placebo and treatment arms were comparable with blood eosinophil counts of approximately 240 cells per μL, exhaled nitric oxide of approximately 25 parts per billion, serum periostin of approximately 58 ng/mL, serum CCL13 of approximately 230 pg/mL, serum CCL17 of approximately 390 pg/mL, and serum total IgE of approximately 420 IU/mL.
We analysed the mean change in biomarker levels from baseline for each lebrikizumab treatment group versus placebo. Comparisons between the 125 mg and 37.5 mg lebrikizumab groups and the placebo group were evaluated using a mixed-effect repeated measures model, and different contrasts were tested. Multiplicity controls were performed to address the issue of multiple testing.
In both studies, there was a significant drop in all four biomarkers measured (p<0.01). Except for serum IgE, the drop was noted within 1 week and continued to drop over the duration of the study, as depicted in Figure 1 and Figure 2.
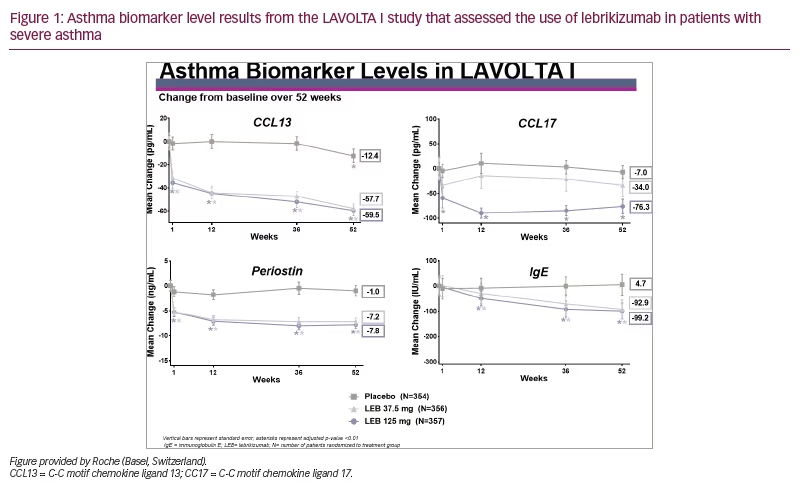
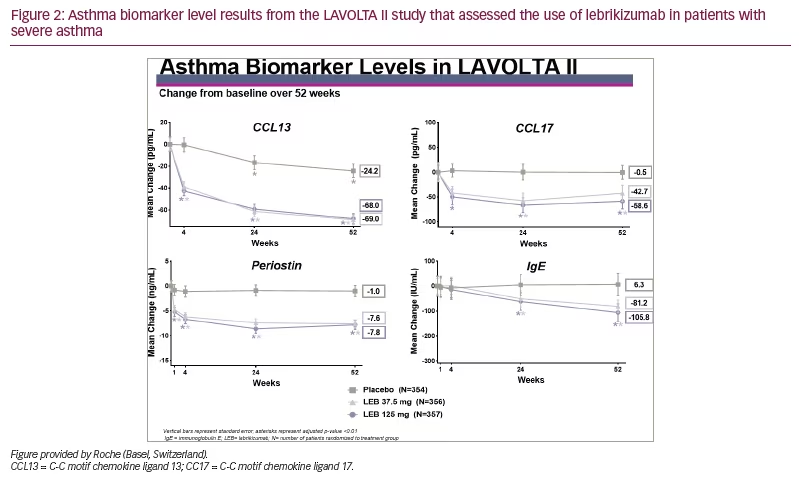
The drop in biomarker levels was significant and approximately equal for both doses of lebrikizumab but slightly greater for the highest dose of lebrikizumab. The drop in IgE was slower compared with the other biomarkers, probably due to the longer half-life of IgE. From this analysis, we concluded that in these phase III studies, treatment with lebrikizumab led to decreased levels of all four circulating inflammatory biomarkers in patients with uncontrolled asthma.
We noted several limitations to this study. First, only circulating serum biomarker levels were measured. Second, the doses used in the LAVOLTA studies were lower than those used in the recent phase III ADvocate studies for atopic dermatitis (ClinicalTrial.gov identifiers: NCT04146363; NCT04178967).7–9
Q. What is the potential clinical significance of these findings?
The results of these studies provide some interesting insights into the potential mechanisms of lebrikizumab in reducing inflammation that could be associated with severe asthma. It will be important to determine the differences in response to the various asthma biologics among patients with severe asthma to develop a precision medicine approach to management. This study offers insight into some unique mechanisms that need to be evaluated for the other asthma biologics. Perhaps, lebrikizumab affects pathways not covered by these other asthma biologics and could lead to a differential response in certain patients. Future studies will aim to test whether lebrikizumab also affects type 2 inflammatory biomarker levels in adolescent and adult patients with atopic dermatitis.